Abstract
Aims
Migraine is the most common disabling headache disorder and is characterized by recurrent throbbing head pain and symptoms of photophobia, phonophobia, nausea, and vomiting. Rimegepant 75 mg, an oral lyophilisate calcitonin gene-related peptide antagonist, is the first treatment approved for both the acute and preventative treatment of migraine, and the first acute therapy approved in over 20-years. The objective was to assess the cost-utility of rimegepant compared with best supportive care (BSC) in the UK, for the acute treatment of migraine in the adults with inadequate symptom relief after taking at least 2 triptans, or for whom triptans are contraindicated or not tolerated.
Materials and methods
A de novo model was developed to estimate incremental costs and quality-adjusted life years (QALYs), structured as a decision tree followed by Markov model. Patients received rimegepant or BSC for a migraine attack and were assessed for response (pain relief at 2-h). Responders and non-responders followed different pain trajectories over 48-h cycles. Non-responders discontinued treatment while responders continued treatment for subsequent attacks, with a proportion discontinuing over time. Data sources included a post-hoc pooled analysis of the phase 3 acute rimegepant trials (NCT03235479, NCT03237845, NCT03461757), and a long-term safety study (NCT03266588). The analysis was conducted from the perspective of the UK National Health Service and Personal Social Services over a 20-year time horizon.
Results
Rimegepant resulted in an incremental cost-utility ratio (ICUR) of £10,309 per QALY gained vs BSC, which is cost-effectiveness at a willingness to pay threshold of £30,000/QALY. Rimegepant generated +0.44 incremental QALYs and higher incremental lifetime costs (£4,492). Improved QALYs for rimegepant were a result of less time spent with severe and moderate headache pain.
Conclusion
This study highlights the economic value of rimegepant which was found to be cost-effective for the acute treatment of migraine in adults unsuitable for triptans.
Introduction
Migraine is a disabling neurologic disease characterized by recurrent attacks of unilateral throbbing head pain, and associated with symptoms of photophobia, phonophobia, nausea, and vomitingCitation1–3. Migraine is the most common disabling headache disorder, with the 2019 global age-standardized prevalence estimated at 14.1%Citation4,Citation5. Migraine prevalence reaches its peak during prime employment years (between the ages of 25 and 55), and disproportionally affects women, who are 2–3 times more likely than men to suffer from migraineCitation4,Citation6,Citation7.
The prevalent, chronic, and disabling nature of migraine poses a significant burden on employers, families, patients, and society through direct costs to the health care system and indirect costs such as lost work productivity, absenteeism, and unemploymentCitation8–10. National Health Service (NHS) in the UK spends an estimated £150 million per year treating patients with migraineCitation8, and in 2018, the annual cost of lost productivity due to migraine was estimated at £8.8 billionCitation9. The economic and humanistic burden of patients unsuitable for acute treatment with triptans is particularly highCitation11–17.
Migraine is characterized by high individual variability, and the optimal approach to treatment varies from person to person. Most patients with migraine require a combination of pharmacotherapy and lifestyle adjustments to effectively manage their conditionCitation3. The British Association of the Study of Headache Guidelines state patients who require acute migraine treatment should be prescribed triptans as first line therapy in a stratified treatment approach, either alone or in combination with simple analgesics such as ibuprofen, aspirin, or paracetamol ()Citation18. However, some patients may not achieve adequate symptom control with triptans due to lack of efficacy or intolerable side effects, and a significant proportion have contraindications which can include a wide range of cardiovascular diseasesCitation19–22. This unmet need has been consistently demonstrated in clinical practice and trial data showing that new triptan users have relatively low persistenceCitation19–22. Inadequate treatment can lead to disease progression, (i.e. migraine chronification), and medication-overuse headache (MOH), whereby patients suffer rebound headaches brought on by their current acute treatmentCitation23–31.
Figure 1. Clinical pathway of care for treatment of acute migraine. Abbreviations. AEs, adverse events; BSC, best supportive care; NSAIDs, non-steroidal anti-inflammatory drugs. 1Consider an anti-emetic in addition to other acute treatment for migraine even in the absence of nausea and vomiting. 2When prescribing a triptan, start with the one with the lowest acquisition cost. Adapted from NICE CG150.
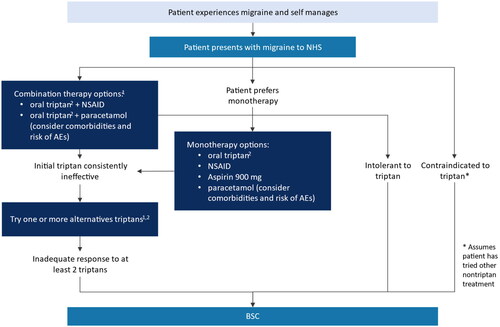
Despite this demonstrated unmet need, there have been no new acute treatments approved for migraine since triptans were licensed in the 1990sCitation32–34. At the time of model development and writing, patients who discontinue ≥2 triptans in the UK have no other pharmaceutical options ()Citation18. Best supportive care (BSC) for these patients consists of lifestyle changes (e.g. trigger avoidance, use of migraine diaries, regular exercise, hydration, and nutrition) and complementary or alternative therapies such as acupuncture and cognitive behavioral therapyCitation3,Citation18. Additionally, there is evidence that in the absence of effective pharmacologic treatments, patients may also resort to taking previously tried and ineffective treatments, that do not provide optimal symptom managementCitation35,Citation36.
Rimegepant, an oral lyophilisate, calcitonin gene-related peptide (CGRP) receptor antagonist is approved for the acute treatment of migraine with or without aura in adults at a recommended dose of 75 mg as needed to treat migraines as they occur (PRN), and as a preventative treatment of episodic migraine in adults who have at least four migraine attacks per month, at a recommended dose of 75 mg every other day (EOD)Citation37. In 2023, rimegepant received positive reimbursement decision for both the acute treatment and prevention of migraine by NICE and SMCCitation38–40.
The current treatment paradigm for migraine separates acute and preventative therapies, as prior to rimegepant, there has not been a therapy effective for both treatment strategies. The focus of this manuscript is PRN treatment for the acute indication, supported by a post-hoc pooled analysis of data from the Phase 3 single attack studies (BHV3000-301 [NCT03235479], BHV3000-302 [NCT03237845], and BHV3000-303 [NCT03461757]). Analyses were performed on subgroups of the pooled population based on prior triptan experience, including triptan naïve patients, current triptan users, and those that had discontinued 1 triptan and ≥2 triptans due to insufficient response (e.g. lack of efficacy and/or poor tolerability)Citation41. The results demonstrated rimegepant provides statistically significant improvements to patients regardless of their previous triptan experience, including those who had discontinued ≥2 triptansCitation41. Additionally, a 52-week open-label study (BHV3000-201 [NCT03266588]) informed discontinuation rates for rimegepant, and demonstrated a reduction in monthly migraine days (MMD) observed for patients using long-term rimegepant acute treatment PRNCitation42,Citation43.
The objective of this study was to assess the cost-utility of rimegepant 75 mg PRN compared with best supportive care (BSC) in the acute treatment of adults with migraine in the UK who have inadequate response to ≥2 triptans, or who have triptan intolerance or contraindication (henceforth referred to as ≥2 triptan discontinuers).
Methods
Model overview
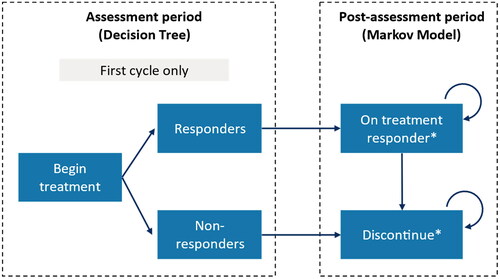
A de novo economic model structured as an initial decision tree followed by a Markov model was developed to assess the cost-utility of rimegepant 75 mg PRN compared with BSC for patients with ≥2 triptans discontinuations ()Citation34. The analysis was conducted from the perspective of the NHS and Personal Social Services. The model structure was informed by an economic evaluation of novel acute therapies in migraine conducted in the US by the Institute for Clinical and Economic Review (ICER) adapted to the UK setting, and to directly incorporate rimegepant trial dataCitation44,Citation47.
Table 1. Features of rimegepant acute treatment migraine model structure.
Model structure
The model structure is consistent with the acute migraine clinical care pathway for the UK (). BSC, using the placebo arm of the trials as a proxy, was chosen as the comparator as at the time of the economic analysis, no other comparators (e.g. lasmiditan, ubrogepant) were available in the UK, for ≥2 triptan discontinuers. Use of a trial-based comparator (e.g. placebo/BSC) allowed for trial data to be used directly in the model. The availability of comprehensive patient-level data also facilitated a responder-based analysis, assuming patients who did not achieve a threshold response for their first migraine would discontinue due to lack of efficacy, and these patients could be explicitly identified within the trial data and pain trajectories calculated accordingly.
The decision tree component of the model included the assessment period, where all patients experienced their first migraine attack (i.e. in the first model cycle only) and received either rimegepant or BSC for treatment of one attack. The patients were then assessed for response (defined as pain relief at 2 h, per expert feedbackCitation48) based on post-hoc pooled efficacy data from the rimegepant acute treatment trials among ≥2 triptan discontinuers (). Response to the first migraine event was used to determine whether patients remained on or discontinued treatment in the model. Patients who did not have a response to rimegepant or BSC were assumed to discontinue their treatment and experience pain trajectories observed for BSC non-responders, based on data from the pooled subgroup analysis of studies BHV3000-301, BHV3000-302, and BHV3000-303. Patients who met the response threshold (pain relief at 2 h) experienced the responder pain trajectories for the relevant treatment arm. Responders were then assumed to continue to respond to treatment when subsequent attacks occurred in following model cycles. In post-assessment period model cycles, the proportion of the cohort with and without migraine was calculated for each 48-h model cycle using baseline MMD frequency distributions.
Figure 2. Overview of the model structure for acute treatment of migraine. *Background mortality included as a separate state.
For BSC patients, it was assumed that the treatment effect would dissipate for BSC responders after 1-year, and they would follow the pain trajectory of BSC non-responders going forward. This is consistent with assumptions of prior technology appraisals in the prevention setting, which concluded that a placebo response should last no longer than 1-yearCitation49,Citation50. Clinical consultation suggested that in real world clinical practice, the placebo effect of acute treatment would be unlikely to last longer than 6-months, however 12-months was implemented in the model as a conservative approach.
A proportion of rimegepant patients who had initially responded to treatment discontinued treatment each cycle (informed by discontinuation patterns observed in ≥2 triptan discontinuers in the long-term safety study BHV3000-201). For parity across treatment arms, the rimegepant discontinuers were also assumed to achieve the benefits of BSC responders for 1 year before transitioning to the outcomes of a BSC non-responder.
Rimegepant is unique, in that it has proven efficacy and safety as both an acute (PRN dosing) and preventative migraine therapy (EOD dosing), at the same 75 mg dose strengthCitation51,Citation52. In the 52-week safety study (BHV3000-201), preventative effects of rimegepant (e.g. reduction in MMDs) were observed in patients taking it PRN when rimegepant was taken at a high enough frequency as an acute treatment (PRN; full 52-weeks of follow up, n = 1,514)Citation43. Based on this evidence, MMD frequency reduction is included in the modelCitation43. A post-hoc regression analysis using patient-level data from study BHV3000-201 determined that ≥2 triptan discontinuers with a MMD frequency greater than eight would experience a reduction in MMD when rimegepant was used consistently as a long-term acute treatment (Supplementary Appendix 2).
No excess mortality was thought to be associated with migraine, so patients in all model states had an equal risk of transitioning to all-cause mortality, which was based on UK life tablesCitation53. An annual discount rate of 3.5% was applied to both costs and benefits, and costs were estimated in 2022 UK pounds ().
In the long-term safety study (BHV3000-201), patients remained on treatment for up to 52-weeks, with only a small percentage discontinuing (9.7% annually). The current model extrapolates these data demonstrating that some patients remain on treatment at 20-years. A lifetime horizon (capped at 20-years) was deemed the most appropriate to capture the cost-utility implications of taking acute treatment per episode, treatment discontinuation over time, and potential costs and benefits of long-term acute treatment with rimegepant ().
Table 2. Summary of model inputs, settings, and rationale.
Though migraine frequency tends to decline with older age, migraine is a chronic diseaseCitation54–56. In a large global survey of patients with migraine (mean age 39.4 years) 49% of respondents reported experiencing migraine attacks for over 10 years, and 27% for more than 20 yearsCitation57. This time horizon was further supported by trial population demographicsCitation51,Citation58,Citation59, clinician insights, and patient testimonialCitation60. In the pivotal clinical trials (BHV3000-301, BHV3000-302, BHV3000-303), on average disease onset was 21 years old, and average age at enrolment was approximately 39 years of ageCitation51,Citation58,Citation59. In a blinded online survey of 164 general practitioners in the UK (conducted as part of the expert validation process for the present analysis), 112/164 (68.3%) stated that patients suffer with migraine for >5 years, 75/164 (45.7%) for >10 years, and 35/164 (21.3%) for >20 years of their lifetimeCitation60. In a similar survey of 12 UK based neurologists, pain specialists and primary care specialists, 10/12 (83.3%) believed that patients suffer with acute migraine attacks for >10 years, and 6/12 (50.0%) for >20 yearsCitation60. During the NICE appraisal process, 86 members of the public wrote to NICE, 15 of whom mentioned suffering with migraine long-term noting they experienced migraine for 20–72 yearsCitation60. The most recent HTAs in migraine prevention have also modelled lifetime time horizonsCitation50,Citation63. The effect of modelling a shorter time horizon (10 years) was explored in scenario analyses.
The cycle length was 48 h, which is a typical trial duration seen in trials of acute migraine therapies, and no half-cycle corrections were applied ().
Model inputs
This economic model was informed by three pivotal, multicentre, Phase 3, single-dose, placebo-controlled studies (BHV3000-301, BHV3000-302, BHV3000-303) which demonstrated that oral rimegepant 75 mg can provide rapid and durable benefits in the acute treatment of migraine, with low rates of adverse events (AEs)Citation51,Citation58,Citation59. A post-hoc pooled analysis of phase 3 single attack trial data confirmed that rimegepant was effective in the target population of ≥2 triptan discontinuersCitation41. In this analysis, rimegepant was more effective than placebo for the coprimary endpoints of pain freedom at 2 h (20.0% vs 10.2%, p = .013) freedom from most bothersome symptom at 2 h (43.0% vs 21.5%, p < .001), and a broad range of secondary endpoints, including pain relief (69.5% vs 36.6%, p < 0.001)Citation41.
As described above, a 52-week safety study (BHV3000-201) was used to inform rates of long-term rimegepant discontinuation and to model MMD reduction observed among long-term users of rimegepant as an acute therapyCitation42,Citation43. The rimegepant clinical trials that informed the current economic analysis were conducted with approval from a formal ethics review committee and with written consent from the patients.
An additional study, BVH3000-310 (NCT04574362), was conducted to further evaluate the safety and efficacy of rimegepant acute treatment; however, this study was not included in the base case analysis because it enrolled adults from China and Korea which is not representative of the UK population. Furthermore, the subgroup of people who had discontinued ≥2 triptans were not available to extract, as triptans are not widely used in China for acute treatment of migraine.
In addition to trial data, inputs were derived from published literature and publicly available sources (). Where data were not available, assumptions were made in the economic analysis as supported by clinical input, previous technology assessments, and real-world evidence (). Key decisions were taken to an advisory board comprised of neurologists and general practitioners, to establish the national clinical consensus where uncertainty or data paucity prohibited a clear view.
Table 3. Key assumptions in the acute economic model.
Baseline patient characteristics
The baseline patient characteristics were from those with ≥2 triptan discontinuations from the long-term safety study BHV3000-201 ()Citation41. For comparison, the mean age-, sex- and baseline MMDs are also presented in for the pooled phase 3 rimegepant trials, stratified by triptan response status. Age and sex distribution was used to calculate background mortality based on UK life tables. MMD distribution was obtained from the long-term safety study BHV3000-201, which was assumed to be representative of the MMD distribution in the UK population. Baseline characteristics from the phase 3 acute RCTs were not used in the model, as these trial inclusion criteria restricted enrolment to patients with two to eight migraine attacks per month. Such inclusion criteria are common in trials of acute migraine treatment; limiting the frequency of MMDs helps avoid confounding efficacy assessment of a single migraine attack that would otherwise be caused by subsequent migraine attacks in patients with higher MMDs. However, it means the migraine frequency distribution of the acute attack trials is not representative of that seen in routine clinical practice.
Table 4. Baseline patient characteristics, pooled across acute trials of rimegepant and stratified by triptan discontinuation status.
Treatment efficacy
Pain relief at 2 h was used in the model to define treatment response, and response rates of 69.6% for rimegepant and 36.7% for BSC were reported for ≥2 triptan discontinuers in the post-hoc pooled analysis of acute treatment trials (). Pain relief at 2 h (defined as initial pain of moderate to severe intensity reduced to mild or no intensity), is both clinically relevant and of importance to patients, was supported by expert feedback from two advisory boardsCitation48 ,and is consistent with how rimegepant will be used in clinical practice. Clinical experts suggested that patients would reasonably expect a therapy to have some pain relief outcome within two hours to be considered effective and would therefore continue utilizing treatment as needed for subsequent migraine attacks. Model results were generated for each baseline MMD value, and a weighted average of results was taken using the BHV3000-201 baseline MMD distribution for patients who had discontinued ≥2 triptans. From the second model cycle onwards, the average probability of experiencing migraine during a 48-h migraine cycle was calculated based on baseline MMD (e.g. patients with 9.2 MMD at baseline would have a 0.605 probability of experiencing a migraine during each 48-h cycle, 9.2 MMD/(365 days/12 months) × 2 days).
Pain trajectories
The efficacy of rimegepant was primarily characterized by improved pain trajectories per migraine event within treatment responders, resulting in less time over 48 h spent in severe and moderate pain and more time spent in mild or no pain. This produces higher utility values on average across a 48-h migraine cycle and additional quality-adjusted life hours (QALH). QALHs can be interpreted in a similar way as quality adjusted life years (QALYs), over the shorter time frame, and were deemed to be the best approach for valuing treatment outcomes (pain freedom, mild, moderate, or severe pain), over the course of the 48-h treatment cycle. Prior to any adjustments for utility between attacks, it was assumed that an hour lived with migraine pain freedom is worth 1 QALH, and that an hour of life lived with mild, moderate, or severe pain is worth less than 1, as informed by literature-based utility valuesCitation64.
To evaluate pain severity trajectories, the percentage of participants in each pain state at each time point and the average time spent in each state across treatment arms over 48 h were calculated (; Supplementary Appendix 3). This analysis was conducted in patients who were ≥2 triptan discontinuers and who had data available across all study timepoints. Among 172 total participants (88 rimegepant, 84 BSC), distributions of pain severity at baseline were as follows across treatment arms: rimegepant: 82% moderate, 18% severe; BSC: 70% moderate, 30% severe. To account for these differences between treatment arms at baseline, a covariate for baseline migraine severity was included in the QALH regression model (Supplementary Table 8). At 2 h, only 2% of participants in the rimegepant arm had severe pain (20% moderate), while 19% of participants in the BSC arm had severe pain (39% moderate; ). By 24 and 48 h, these values drastically decreased in both arms, although more so in the rimegepant arm. For rimegepant, severe pain was seen in 0% of participants at 24 h (5% moderate) and 0% at 48 h (3% moderate; ). For BSC, severe pain was seen in 6% of participants at 24 h (14% moderate) and 5% at 48 h (13% moderate; ).
Figure 3. Patient pain trajectories for ≥2 triptan discontinuers treated with rimegepant or placebo in Studies BHV3000-301, -302-, 303.
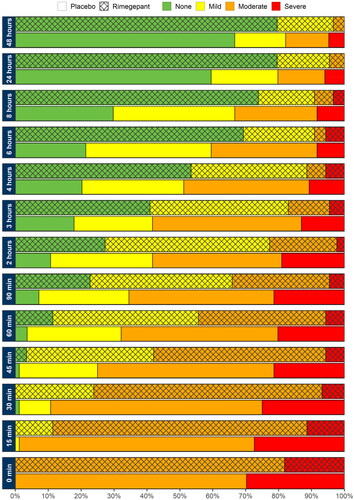
Averaging across all participants, the mean (SD) time spent with no pain over 48 h was higher for rimegepant (34.91 h [14.46 h]) than it was for BSC (23.28 [17.30]). Likewise, rimegepant participants spent less time in severe pain over 48 h (0.64 [2.53]) compared to BSC participants (3.43 [8.59]). The mean vs median QALH values for the data underlying the QALH regression analysis is included in Supplementary Table 7. Trends in responders and non-responders and rimegepant vs BSC were consistent for both means and medians.
MMD Reduction with rimegepant PRN dosing
Rimegepant PRN was found to be associated with a reduction in MMD when taken in high enough frequency (>8 MMD for ≥2 triptan discontinuers in study BHV3000-201). Whilst this is based on single arm trial data, this preventative effect from higher frequency acute use was deemed clinically plausible by clinical experts in the context rimegepant’s dual indication for acute and preventative treatment. Therefore, to predict MMD reductions associated with long-term use of rimegepant as an acute treatment, a regression analysis for change from baseline in MMD was conducted using patient-level data from the PRN dosing groups in the BHV3000-201 study (Supplementary Appendix 2). Regression analyses for number of migraine events in the base case were all fit to the patient population receiving acute treatment. Model covariates included the following: baseline MMD, prior triptan lines (naïve, 1, or 2+), the number of pills taken per MMD, and if the patient used prophylactic migraine medication (Yes or No). Results from the regression indicated that patients having greater MMD at baseline, and thus taking more frequent acute doses of rimegepant, were more likely to experience preventative benefits (e.g. MMD reduction). Therefore, this reduction in MMD applied at a frequency of greater than eight doses per month (corresponding to patients with >8 MMDs). At MMD below this level, no preventative effect was modelled, and the MMD frequency in rimegepant and BSC patients was assumed to be equal.
Treatment discontinuation
Assessment period
Patients who did not respond to rimegepant treatment (based on pain relief at 2 h), follow an untreated trajectory (BSC non-responders) immediately after the first migraine event. The trajectory of BSC non-responders also trended towards pain freedom and relief over 48 h as per the pooled acute trial results, but less rapidly than BSC responders or rimegepant responders or non-responders, reflecting the typical resolution of a migraine without effective treatment. The rimegepant non-responders were assumed to incur the cost of one full 8-pack of rimegepant prior to discontinuing, which is a conservative estimate given that patients may use a full package before discontinuing due to lack of response.
Post-assessment period
Long-term discontinuation in the post assessment period was informed by the subset of patients (responders with ≥2 triptan discontinuations) from the post-hoc pooled rimegepant acute treatment studies who continued into the long-term safety study (BHV3000-201). A discontinuation rate of 9.7% over 1 year was applied. Because of the memoryless property of a Markov model and the fact that discontinuation happens at varying time points throughout the time horizon, it is not possible to track time since discontinuation in order to identify whether a patient had discontinued within the past 12 months. To address this the adjustment to treatment benefits for 12 months post-discontinuation (which is applied to achieve parity with placebo responders) is achieved by a one-off application of the associated QALY difference between placebo responders vs. non-responders for 12 months’ worth of migraine events at the time of discontinuation, adjusted for mortality and any relevant time horizon cap over the subsequent 12 months.
Utility values
During a migraine attack, pain severity is thought to be the driving factor of health-related quality of life (HRQoL), however HRQoL is also negatively affected on migraine free days, due in part to the unpredictability of migraine, anticipation of the next attack, and lifestyle changes made to avoid triggersCitation72,Citation73. Therefore, utilities are incorporated into the model in two distinct ways: (1) by applying an interictal (between attack) utility value for patients not experiencing an attack during any given 48-h cycle, based on MMD frequency at baseline, and (2) by deriving the overall QALH per migraine event, per treatment arm by applying pain state utilities to patient pain trajectories (Supplementary Appendix 3).
Baseline and interictal utility values
Baseline utility values were generated from migraine-specific quality of life questionnaire version 2 (MSQv2) data at baseline and throughout the BHV3000-201 study, mapped to EQ-5D using a validated algorithm for episodic migraineCitation62. To estimate interictal utility values, a mixed model repeated measures regression model was fit. A model without baseline EQ-5D and baseline MMD (while still using baseline + post-baseline data) included as model covariates was chosen because the cost-utility model was designed to explore populations with varying baseline MMD levels, which in turn, would impact expected baseline EQ-5D and result in a circular model structure. The chosen model incorporated the following covariates: age, sex, triptan lines, and absolute MMD. The resulting utilities were applied at baseline and during model cycles for which a migraine event did not occur. Each MMD averted was associated with an increment of 0.0054 to utility ().
Migraine event utilities
For migraine event utilities, data from Stafford et al.Citation64 were selected from a systematic literature review for the base case, due to their relevance and generalizability to the UK population. While utility values from a study by Xu et al. were also considered, there were concerns regarding the face validity of the severe pain value (0.44)Citation74. For modelling a real-world migraine population, the value from Xu et al. for severe migraine pain was considered implausibly high (as validated in the UK advisory board).
In Stafford et al.Citation64, the utility value for severe migraine pain was estimated at −0.20, a negative number indicating a state worse than death (; Supplementary Table 1). Due to the extreme nature of this utility value, the impact of this was explored in a scenario analysis, where the severe pain level was set to 0. It was anticipated a priori that this value would have a limited impact on results as, over the 48-h observation period in the rimegepant post-hoc pooled acute treatment trials, the time spent on the highest pain intensity “severe pain” was relatively short compared to the three other categories.
To calculate QALHs from pain trajectories, the time per pain category (none, mild, moderate, severe; ) was multiplied by health state utilities derived from Stafford et al. and then summed over the 48-h study period to generate QALH over 48 hCitation64. A regression analysis was then fitted to describe QALH outcomes adjusted for treatment arm, 2-h response status, baseline MMD, and baseline migraine severity. The predicted QALH were adjusted to reflect the baseline utility value. Pain severity utilities were adjusted for consistency with background interictal HRQoL such that the pain-free utility value was set to be equivalent to the background utility value, and the utilities for the remaining health states were multiplicatively adjusted so that the relative distribution of utilities by pain state remained consistent. For further details on QALH derivation, please see Supplementary Appendix 3.
Costs and health care resource use
Costs and health care resource use (HCRU) were based on published sources (). The primary direct medical cost in migraine was the price of treatment. Other costs included those related to general practitioner visits, emergency department visits, and hospitalizations. The drug acquisition cost for rimegepant was £103.20 per 8-pack, for both initial and ongoing treatment. The model conservatively assumed no treatment costs were associated with BSC, despite evidence that some patients would continue to use triptans with suboptimal effectCitation35,Citation36. Unit costs were derived from Personal Social Services Research Unit (PSSRU) and NHS references costs (Supplementary Table 2 and Supplementary Table 3). Costs inputs were all available in 2022 UK pounds therefore no inflation adjustment was required.
For each migraine attack, a probability of incurring costs for each of the HCRU categories was estimated based on HCRU analyzed by MMD frequency in Vo et al., and probabilities were weighted by baseline MMD frequency groups using data from the subgroup who discontinued ≥2 triptans in the BHV3000-201 study (Supplementary Table 2)Citation65. The HCRU probability per-migraine event was only applied to patients who experienced moderate or severe pain at 24 h, consistent with the prior economic analysisCitation44. Based on the post-hoc pooled trial data, 4.55% of rimegepant and 20.24% of BSC patients with ≥2 triptan discontinuations incurred HCRU based on moderate/severe pain at 24 h.
AEs were not included in the model given the low (< 2%) incidence observed in clinical trials.
Model outcomes
Model outcomes were evaluated over a 20-year time horizon in the base case and included total direct medical costs, cumulative QALYs, incremental costs and QALYs between treatments over the modelled time horizon and the incremental cost-utility ratio (ICUR) per QALY gained. The ICUR represents the costs required to obtain one additional QALYCitation75; in the UK this is typically compared to a benchmark willingness to pay of £20,000–£30,000 per QALYCitation46.
A probabilistic sensitivity analysis (PSA) was undertaken to examine the uncertainty surrounding model parameters. The PSA was conducted using 1,000 iterations and results were used to create a scatter plot and cost-effectiveness acceptability curve (CEAC). In addition, deterministic one-way sensitivity analysis (OWSA) was conducted for individual model parameters to evaluate the impact of a given parameter on the ICUR ().
Table 5. Deterministic one-way sensitivity analysis ranges.
Given the recent reimbursement of rimegepant in acute (and prevention) in the UK, the authors sought to replicate the model settings used by the NICE appraisal committee, to aid discussion of the results and explore the impact of various assumptions. Therefore, a scenario analysis was performed using the NICE base case settings, as described in Supplementary Table 4.
Additionally, scenario analyses were performed to investigate the effects of certain model inputs on costs and outcomes. Scenarios included: a discount rate of 1.5%, time horizons of 10 years, response defined as pain relief at 8 h, no reduction of MMD frequency associated with PRN rimegepant use, QALH based on raw data (pain intensity x hour; see Supplementary Appendix 3), additive adjustment for event utility regression (see Supplementary Appendix 3), migraine event utility values with the severe utility set to 0, modified intention-to-treat (mITT) patient population, all-cause discontinuation, immediate transition to BSC non-responders at discontinuation, and BSC waning effects of 6 and 18 months.
Results
Base case results
Total costs, QALYs, and incremental cost per QALY for rimegepant vs BSC are presented in . In the base case analysis, rimegepant was associated with 0.44 incremental QALYs compared with BSC, and the rimegepant total lifetime costs were £4,492 higher than BSC costs. The ICUR was £10,309 per QALY gained.
Table 6. Base-case results acute treatment of migraine.
Sensitivity analyses
Probabilistic sensitivity analysis
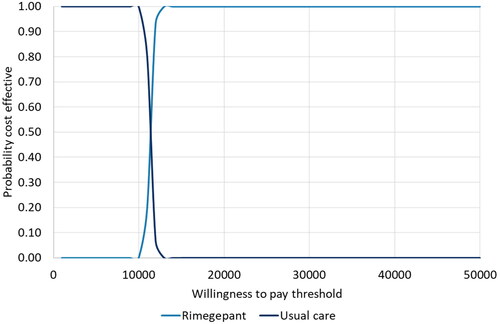
PSA was performed by allowing all parameters to vary according to their sampling distributions. All 1,000 iterations indicated rimegepant provided a clinical benefit vs BSC and was associated with an incremental cost. The scatterplot of incremental cost vs incremental QALYs for rimegepant vs BSC and the 95% credible ellipse is presented in Supplementary figure 1. Compared with BSC, rimegepant generated 0.44 incremental QALYs, and the rimegepant treatment cohort had higher total lifetime costs (). The probabilistic ICUR was £10,337 per QALY gained. The CEAC indicated there is an 100% chance that rimegepant is cost-effective at a willingness-to-pay (WTP) threshold of £30,000 per QALY ().
Table 7. Results of the probabilistic sensitivity analysis for rimegepant vs best supportive care for the acute treatment of migraine.
Deterministic one-way sensitivity analysis
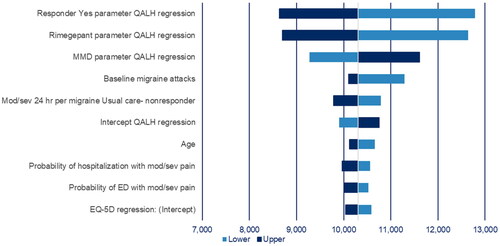
The OWSA for key inputs yielded similar results to the base case. Model results were most sensitive to the parameter values in the QALH regression, with a lower ICUR associated with a higher parameter value for responder rates or the rimegepant coefficient, and lower (negative) parameter value for MMD (i.e. lower numerically but larger in absolute value) (). In the base case QALH regression, these regression coefficients are all associated with a benefit to rimegepant (i.e. direct impact of rimegepant, benefits of response status, and potential for greater improvement in patients with more MMDs at baseline) and amplifying or reducing these respective effects was found to be relatively influential on the ICUR. Results were also relatively sensitive to probability of moderate/severe pain at 24 h for BSC patients, and baseline MMD.
Scenario analyses
The scenario analysis that replicated the NICE settings resulted in the largest ICUR increase, from £10,309/QALY in the base case analysis to £29,833/QALY (). The largest decreases in ICUR were observed for a responder definition of pain relief at eight hours (£5,796), and an additive vs multiplicative adjustment to utilities (£7,984).
Table 8. Scenario analysis: rimegepant vs best supportive care.
The additive adjustment was particularly impactful given the negative utility associated with severe pain, reported by Stafford et al.Citation64 For example, the utility for extreme pain was adjusted to a small positive value by the multiplicate adjustment and small negative value by the additive adjustment. Therefore, additive adjustment resulted in greater benefit and lower ICUR for rimegepant. However, for the scenario analysis in which that negative utility was capped at 0 (for a multiplicative adjustment), the ICUR was relatively close to base case at £11,049. Thus, for the base case setting of multiplicative utility adjustment, the incorporation of a negative utility did not have a notable impact on the ICUR. All remaining scenario analyses were also within approximately +/− £2,000 of the base case value and all were under the £30,000 willingness to pay threshold. This included varying the modelled time horizon to 5 and 10 years, respectively, relative to a base case of 20 years.
Discussion
In the UK, acute treatment with rimegepant was found to be cost-effective compared with BSC with an ICUR of £10,309 per QALY gained, for adult patients who had discontinued ≥2 triptans. Rimegepant improved HRQoL by reducing the amount of time patients spent with severe or moderate headache pain and by reducing MMD frequency compared to BSC. The probability that rimegepant was cost-effective was 100% at a WTP threshold of £30,000 per QALY. Thus, in the context of standard UK thresholdsCitation46, rimegepant was found to be a cost-effective therapy; for consideration of other countries, the analysis can be adapted to reflect local health system practices and costs, to be compared to willingness-to-pay thresholds.
The model was structured as an initial decision tree, followed by a Markov model to characterize time spent in on-treatment vs. off-treatment health states. The structure aligns to anticipated clinical practice, whereby the decision to continue treatment would follow from response status to an initial treatment trial. Given the relatively simple model structure and small number of health states, for the most part the “memoryless” property of the Markov model was not a particular hindrance in modelling the disease process. One exception to this was the potential for an ongoing treatment effect post-discontinuation, included for parity in assumptions with BSC, which required tracking for 12 months post-discontinuation. To address this limitation in approach, a “lump sum” adjustment was made at the time of discontinuation; while a simulation approach could more directly model this process, the lump-sum currently applied accounts for the adjustment to migraine events over the course of 12 months, and incorporates patient mortality, so as such is not anticipated to be a major limitation. The QALHs per migraine by treatment and response status (and the variability reflected in the PSA) incorporate the range of outcomes observed across individual patients in the rimegepant trial program. As such and given that no notable dependencies or non-linearities were observed, a standard cohort-based Markov approach would be sufficient, as opposed to a patient-level simulation approach such as discrete event simulation.
Overall, the cost-utility analysis was found to be generally stable. The mean ICUR from the PSA was £10,337 per QALY gained, which was consistent with the deterministic result. In OWSA, the greatest drivers in the model were the parameter values in the QALH regression, with higher ICURs associated with lower responder rates. Scenario analysis demonstrated that the ICUR was more favourable (£5,796 per QALY) when response was defined as pain relief at 8 h.
The scenario that adopted the NICE committee’s settings generated a larger ICUR, though still within the WTP threshold of £30,000. This increase was driven primarily by the reduction of the time horizon from 20-year to 2-year, use of the mITT population (vs ≥2 triptan discontinuers, for whom rimegepant is indicated), the removal of BSC (placebo) response waning (i.e. assuming an indefinite placebo response), and the complete removal of reduction of MMD with rimegepant PRN use.
Regarding the appropriateness of the 20-year time horizon, evidence suggests that a significant proportion of patients will receive acute rimegepant long-term as migraine is a chronic condition. Despite the peak in prevalence in working aged individuals, migraine impact patients over the course of their lifetimeCitation54–56. Further, due to patterns of treatment adherence/discontinuation, and potential MMD reduction over time, while each dose of rimegepant for acute use is only relevant for a 48-h period, the cost-effectiveness dynamics continue to evolve over an extended time period.
The NICE scenario assumed no waning of BSC response over the 2-year time horizon, which means that BSC patients are assumed to receive clinical improvement without active treatment and with no cost to the NHS during that time (given no BSC costs are included in the model). An indefinite placebo response is clinically implausible and not supported by clinical advice, and artificially increases the BSC QALY gains, with no associated costs. With respect to MMD frequency, the NICE committee acknowledged that there is biological plausibility in the suggestion that taking rimegepant as needed may reduce MMDs, but cited lack of evidence to support the inclusion of this effect in the model at the time of investigation and it should be deemed an uncaptured benefitCitation60. MMD reduction evidence used within the model are based on a long-term safety study demonstrating that repeated treatment with rimegepant PRN impacts frequency of migraine in addition to acute pain managementCitation71. Since the NICE appraisal, further evidence has been generated to support the MMD reduction with PRN useCitation61.
The current model did not account for MOH or migraine chronification which have both been linked to suboptimal acute migraine managementCitation23–31. Unlike triptans and other front-line analgesics, rimegepant has no evidence of MOH, further reducing direct heath care costs. For example, a real world US-based administrative claims analysis (2018–2021) of 423,312 patients with ≥2 rimegepant prescription fills observed a significant decrease in MOH point prevalence in the rimegepant-treated population across all time-pointsCitation77. In summary, rimegepant was deemed cost-effective despite numerous conservative assumptions in both the present base case analysis and in the NICE scenario. This suggests that the true cost-impact to the NHS may be even less than the ICURs reported here, given several assumptions favored BSC and underestimated potential value of rimegepant.
This is the first UK cost-utility analysis of a novel acute therapy for adults with migraine who have discontinued ≥2 triptans, as no novel therapies have been developed for the acute treatment of migraine in the last 20 years. While this is the first economic analysis for rimegepant in the UK setting, a US cost-utility analysis was performed by ICER for CGRP receptor antagonists for the acute treatment of migraineCitation44,Citation47. The current analysis provides additional insight into cost-utility analyses for CGRP receptor antagonists in the current treatment landscape in the UK. The US ICER analysis included comparators not yet available in the UK (lasmiditan and ubrogepant) and given lack of patient-level data availability across comparators, assumptions had to be made in the ICER model to supplement the aggregate published data and network meta-analysis. The present UK analysis included a trial-based comparator of BSC, using the placebo arm of the trials as a proxy, which allowed for trial data to be used directly (i.e. full 48-h pain severity trajectories were available for both rimegepant and BSC). The availability of full patient-level data facilitated a responder-based analysis, assuming patients who did not achieve a threshold response would discontinue due to lack of efficacy, and these patients were explicitly identified within the trial data and pain trajectories refined accordingly.
The current analysis is not without limitations. Treatment efficacy was informed by a subset of patients from the rimegepant acute clinical trial program, who were triptan discontinuers, rather than the entire trial cohort. While this was the population of interest, it reduced the sample size available to inform treatment efficacy and potentially compromised randomization (although no evidence was observed of a resulting patient imbalance on other characteristics), which could be more prone to potential bias. The economic analysis is limited by the single attack study design of the rimegepant acute treatment trials (Study BHV3000-301, -302, and -303) meaning no clinical data available indicating how many initial non-responders would respond after taking rimegepant for a second or third migraine. The model therefore assumed patients who did not respond to the first treatment (based on pain relief at 2 h) would not respond to rimegepant in subsequent attacks and it was assumed that while the cost of one full package of oral lyophilisate would be incurred, no benefit would be realized, and no further packages would be purchased. The model also includes an option for 8-h outcomes to be used as an indicator for response to incorporate later-onset response to therapy; however, 8-h response may be confounded with spontaneous resolution, and it was assumed that 2-h outcomes would be more relevant for predicting patient satisfaction with treatment. Assuming that the same treatment patterns would be followed in a real-world setting, expenditure on rimegepant would be optimized for a responder-based treatment paradigm, as it would only be purchased and utilized within the subset of patients who receive benefit. Patients who do not benefit from rimegepant would then revert to standard of care and not incur costs beyond the one-time purchase of a single package.
Conversely, real-world analyses demonstrate that patients receiving triptans for the treatment of acute migraines often continue treatment when faced with inefficacy or intolerance, due to a lack of effective alternativesCitation35,Citation36. Thus, a substantial unmet need exists for effective treatment options for acute migraine managementCitation36,Citation78. While the present study establishes the cost-effectiveness of rimegepant in the UK at the individual patient level, future research could characterize and quantify the clinical burden faced by this population, facilitating a population-level analysis of the benefits of rimegepant. While this would allow for the magnitude of the clinical and economic benefits to be better understood; regardless, the analysis presented here found that rimegepant presents an economically viable option for a patient population with established burden and unmet need, and a policy of making it available to these individuals would improve overall health outcomes in a population who currently have limited treatment options.
In the UK, there are no clinical stopping rules for patients who have inadequate response, intolerance or who are contraindicated to triptansCitation79. Clinical guidelines for triptans recommend multiple trials of the same triptan or switching to an alternate triptan before stopping treatment. For example, the European Headache Foundation practice guidelines recommend that three attacks be treated at each step prior to proceeding to the next step to achieve cost-effective careCitation79. Based on these existing guidelines for symptomatic and specific migraine therapies, it would be anticipated that in clinical practice, patients would have access to more than one pill after being prescribed rimegepant, and would likely repeat attempts to obtain pain relief on subsequent migraines before stopping treatment.
While there is no direct evidence available examining specific efficacy outcomes across multiple attacks in the acute treatment setting, relatively low discontinuation was observed among patients receiving long-term acute treatment in BHV3000-201, implying that patients continued to derive acute treatment benefit over time, even those who do not meet the responder definition of pain relief at 2 h. For example, amongst patients treated with rimegepant PRN in BHV3000-201, there was a high degree of patient satisfaction (74% completely or very satisfied) and improvement in the Clinical Global Impression of Change score at 52-weeks (90.9%)Citation80. Additional subgroup analyses from BHV3000-201 showed similar results for those with history of ≥1 and ≥2 triptan discontinuations. This again suggests that the current analysis is a conservative assessment of the value of rimegepant as used in clinical practice.
Finally, the current economic analysis is specific to the UK and from the perspective of the NHS, which limits it’s generalizability to other countries. However, we would expect good external validity of the overall findings (e.g. that rimegepant is cost-effective) if analysis were to be adapted to other jurisdictions with similar approaches to migraine management as the UK.
Conclusions
In the UK, rimegepant is considered more cost-effective compared to BSC with an estimated ICUR of £10,309 per QALY among ≥2 triptan discontinuers. Phase 3 trials in this patient population demonstrated significant clinically meaningful responses for rimegepant compared with BSC, which translated into incremental QALYs of 0.44. Rimegepant offers an opportunity for patients with this prevalent neurologic disorder to achieve symptom relief, rapid and sustained return to function, and an enhanced quality of life.
Transparency
Declaration of funding
This study was sponsored by Biohaven which was acquired by Pfizer in October 2022.
Declaration of financial/other relationships
RP, AJ, AA, SL, TT are employed by and hold stock/options in Pfizer.
GJL is employed by and holds stock/options in Biohaven.
KJ, LCP, and EP are employed by Broadstreet HEOR, which was a paid consultant to Pfizer to conduct the study and for the development of this manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (307.7 KB)Acknowledgements
None stated.
References
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202.
- Mayans L, Walling A. Acute migraine headache: treatment strategies. Am Fam Physician. 2018;97(4):243–251.
- Ailani J, Burch R, Robbins M. The American headache society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021–1039. doi: 10.1111/head.14153.
- Safiri S, Pourfathi H, Eagan A, et al. Global, regional, and national burden of migraine in 204 countries and territories, 1990 to 2019. Pain. 2022;163(2):e293–e309. doi: 10.1097/j.pain.0000000000002275.
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2.
- Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–349. doi: 10.1212/01.wnl.0000252808.97649.21.
- Lipton RB, Rm, Munjal S. One-year incidence of migraine in the US population: results from the migraine in America symptoms and treatment (MAST) study (abstract OR08). Headache. 2019;59:9–10.
- National Health Service. Improved NHS migraine care to save thousands of hospital stays. London: NHS; 2020 [cited April 2022]. https://www.england.nhs.uk/2020/01/improved-nhs-migraine-care/
- The Work Foundation. Society’s headache: The socioeconomic impact of migraine: The Work Foundation; 2018 [cited April 2022]. https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/lums/work-foundation/SocietysHeadacheTheSocioeconomicimpactofmigraine.pdf.
- Shapiro RE, Martin AA, Bhardwaj S, et al. Relationships between headache frequency, disability, and disability-related unemployment among adults with migraine. J Manag Care Spec Pharm. 2023;29(2):197–209. doi: 10.18553/jmcp.2023.29.2.197.
- Fischer M, Frank F, Wille G, et al. Triptans for acute migraine headache: current experience with triptan use and prescription habits in a tertiary care headache outpatient clinic: an observational study. Headache. 2016;56(6):952–960. doi: 10.1111/head.12820.
- Wells RE, Markowitz SY, Baron EP, et al. Identifying the factors underlying discontinuation of triptans. Headache. 2014;54(2):278–289. doi: 10.1111/head.12198.
- Holland S, Fanning KM, Serrano D, et al. Rates and reasons for discontinuation of triptans and opioids in episodic migraine: results from the American migraine prevalence and prevention (AMPP) study. J Neurol Sci. 2013;326(1-2):10–17. doi: 10.1016/j.jns.2012.12.020.
- Hirata K, Ueda K, Komori M, et al. Unmet needs in Japanese patients who report insufficient efficacy with triptans for acute treatment of migraine: retrospective analysis of real-world data. Pain Ther. 2021;10(1):415–432. doi: 10.1007/s40122-020-00223-y.
- Lombard L, Farrar M, Ye W, et al. A global real-world assessment of the impact on health-related quality of life and work productivity of migraine in patients with insufficient vs good response to triptan medication. J Headache Pain. 2020;21(1):41. doi: 10.1186/s10194-020-01110-9.
- Marcus SC, Shewale AR, Silberstein SD, et al. Comparison of healthcare resource utilization and costs among patients with migraine with potentially adequate and insufficient triptan response. Cephalalgia. 2020;40(7):639–649. doi: 10.1177/0333102420915167.
- Korolainen M, Kurki S, Lassenius M, et al. Burden of migraine in Finland: health care resource use, sick-leaves and comorbidities in occupational health care. J Headache Pain. 2019;20(1):13. doi: 10.1186/s10194-019-0964-5.
- British Association for the Study of Headache. National headache management system for adults. 2019 [2024 Apr 11]. Available from: https://headache.org.uk/wp-content/uploads/2023/02/bash-guideline-2019.pdf
- Harris L, L'Italien G, O'Connell T, et al. A framework for estimating the eligible patient population for new migraine acute therapies in the United States. Adv Ther. 2021;38(10):5087–5097. doi: 10.1007/s12325-021-01781-z.
- Lipton RB, Reed ML, Kurth T, et al. Framingham‐based cardiovascular risk estimates among people with episodic migraine in the US population: results from the American migraine prevalence and prevention (AMPP) study. Headache. 2017;57(10):1507–1521. doi: 10.1111/head.13179.
- Buse DC, Reed ML, Fanning KM, et al. Cardiovascular events, conditions, and procedures among people with episodic migraine in the US population: results from the American migraine prevalence and prevention (AMPP) study. Headache. 2017;57(1):31–44. doi: 10.1111/head.12962.
- Lipton RB, Buse DC, Serrano D, et al. Examination of unmet treatment needs among persons with episodic migraine: results of the American migraine prevalence and prevention (AMPP) study. Headache. 2013;53(8):1300–1311. doi: 10.1111/head.12154.
- Limmroth V, Katsarava Z, Fritsche G, et al. Features of medication overuse headache following overuse of different acute headache drugs. Neurology. 2002;59(7):1011–1014. doi: 10.1212/wnl.59.7.1011.
- Dodick DW, Doty EG, Aurora SK, et al. Medication overuse in a subgroup analysis of phase 3 placebo-controlled studies of galcanezumab in the prevention of episodic and chronic migraine. Cephalalgia. 2021;41(3):340–352. doi: 10.1177/0333102420966658.
- Schwedt TJ, Alam A, Reed ML, et al. Factors associated with acute medication overuse in people with migraine: results from the 2017 migraine in America symptoms and treatment (MAST) study. J Headache Pain. 2018;19(1):38. doi: 10.1186/s10194-018-0865-z.
- Schwedt TJ, Hentz JG, Sahai-Srivastava S, et al. Headache characteristics and burden from chronic migraine with medication overuse headache: cross-sectional observations from the medication overuse treatment strategy trial. Headache. 2021;61(2):351–362. doi: 10.1111/head.14056.
- May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol. 2016;12(8):455–464. doi: 10.1038/nrneurol.2016.93.
- Mungoven TJ, Henderson LA, Meylakh N. Chronic migraine pathophysiology and treatment: a review of current perspectives. Front Pain Res. 2021;2:705276. doi: 10.3389/fpain.2021.705276.
- Rothrock JF. Chronic migraine: medication overuse headache. Headache. 2007;47(3):467–468. doi: 10.1111/j.1526-4610.2007.00748.x.
- Torres-Ferrús M, Ursitti F, Alpuente A, et al. From transformation to chronification of migraine: pathophysiological and clinical aspects. J Headache Pain. 2020;21(1):42. doi: 10.1186/s10194-020-01111-8.
- Deighton AM, Harris LA, Johnston K, et al. The burden of medication overuse headache and patterns of switching and discontinuation among triptan users: a systematic literature review. BMC Neurol. 2021;21(1):425. doi: 10.1186/s12883-021-02451-x.
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the quality standards subcommittee of the American academy of neurology. Neurology. 2000;55(6):754–762. doi: 10.1212/wnl.55.6.754.
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the a merican H eadache S ociety evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3–20. doi: 10.1111/head.12499.
- National Institute for Health and Clinical Excellence. Headaches in over 12s: diagnosis and management: clinical guideline (CG150). Manchester: NICE; 2012 [cited May 2021]. https://www.nice.org.uk/guidance/cg150/chapter/Key-priorities-for-implementation#tensiontype-headache-migraine-and-cluster-headache
- Gendolla A, Rauer N, Kraemer S, et al. Epidemiology, demographics, triptan contraindications, and prescription patterns of patients with migraine: a German claims database study. Neurol Ther. 2022;11(1):167–183. doi: 10.1007/s40120-021-00304-w.
- Piccinni C, Cevoli S, Ronconi G, et al. A real-world study on unmet medical needs in triptan-treated migraine: prevalence, preventive therapies and triptan use modification from a large Italian population along two years. J Headache Pain. 2019;20(1):74. doi: 10.1186/s10194-019-1027-7.
- European Medicines Agency. Summary of product characteristics: VYDURA 75 mg oral lyophilisate 2023 [cited 2023 Nov 24]. https://www.ema.europa.eu/en/documents/product-information/vydura-epar-product-information_en.pdf.
- National Institute for Health and Care Excellence. Rimegepant for treating migraine (TA919) 2023 [cited 2023 Oct 23]. https://www.nice.org.uk/guidance/TA919.
- National Institute for Health and Care Excellence. Rimegepant for preventing migraine (TA906). 2023. https://www.nice.org.uk/guidance/TA906.
- Scottish Medicines Consortium. Rimegepant (Vydrua®) is accepted for restricted use within NHS Scotland. 2023 [cited 2023 Nov 23]. https://www.scottishmedicines.org.uk/medicines-advice/rimegepant-vydura-resub-smc2603/.
- Lipton RB, Blumenfeld A, Jensen CM, et al. Efficacy of rimegepant for the acute treatment of migraine based on triptan treatment experience: pooled results from three phase 3 randomized clinical trials. Cephalalgia. 2023;43(2):3331024221141686. doi: 10.1177/03331024221141686.
- Lipton RB, Berman G, Kudrow D, et al. Long-term, open-label safety study of rimegepant 75 mg for the treatment of migraine (study 201): interim analysis of safety and exploratory efficacy (poster P235LB). Headache. 2019;59(S1):1–208.
- L’Italien G, Popoff E, Johnston K, et al. Rimegepant 75 mg for acute treatment of migraine is associated with significant reduction in monthly migraine days: results from a long-term, open-label study. Cephalalgia Rep. 2022;5:251581632210755. doi: 10.1177/25158163221075596.
- Atlas S, Touchette D, Agboola F, et al. Acute treatments for migraine: effectiveness and value. 2020 [cited 2022 Jan 18]. http://icer-review.org/material/acute-migraine-evidence-report/.
- Biohaven Pharmaceuticals Inc. Data on file: clinical study report BHV3000-303: a Phase 3, Double-blind, Randomized, Placebo-controlled, Safety and Efficacy Trial of BHV-3000 (rimegepant) Orally Disintegrating Tablet (ODT) for the Acute Treatment of Migraine. 2019.
- National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal 2013. Manchester: NICE; 2013. https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781
- Touchette D, Atlas S, Agboola F, et al. Pnd15 long-term cost-effectiveness of lasmiditan, ubrogepant and rimegepant for treatment of acute migraine. Value Health. 2020;23: S261. doi: 10.1016/j.jval.2020.04.908.
- Diener H, Holle-Lee D, Nagel S, et al. Treatment of migraine attacks and prevention of migraine: guidelines by the German migraine and headache society and the German society of neurology. Clin Transl Neurosci. 2019;3:1–40. doi: 10.1177/2514183X18823377.
- National Institute for Health and Clinical Excellence. Erenumab for preventing migraine: Technology appraisal guidance (TA682). Manchester: NICE; 2021 [cited Apr 2022]. https://www.nice.org.uk/guidance/ta682/resources/erenumab-for-preventing-migraine-pdf-82609376694469.
- National Institute for Health and Clinical Excellence. Fremanezumab for preventing migraine technology appraisal guidance (TA764). Manchester: NICE; 2022 [cited Apr 2022]. https://www.nice.org.uk/guidance/ta764
- Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394(10200):737–745. doi: 10.1016/S0140-6736(19)31606-X.
- Croop R, Lipton RB, Kudrow D, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397(10268):51–60. doi: 10.1016/S0140-6736(20)32544-7.
- Office for National Statistics. National life tables, United Kingdom 2018-2020. 2021 [cited 2022 Jan 2022]. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2018to2020.
- Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol. 2017;16(1):76–87. doi: 10.1016/s1474-4422(16)30293-9.
- Faubion SS, Batur P, Calhoun AH. Migraine throughout the female reproductive life cycle. Mayo Clin Proc. 2018;93(5):639–645. doi: 10.1016/j.mayocp.2017.11.027.
- Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries; 2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6.
- Martelletti P, Schwedt TJ, Lanteri-Minet M, et al. My migraine voice survey: a global study of disease burden among individuals with migraine for whom preventive treatments have failed. J Headache Pain. 2018;19(1):115. doi: 10.1186/s10194-018-0946-z.
- Lipton RBC, Stock EG, Stock D. et al. Efficacy, safety, and tolerability of rimegepant 75 Mg, an oral CGRP receptor antagonist, for the acute treatment of migraine: results from a double-blind, randomized, placebo-controlled trial, study, 301. Headache. 2018;58(8):1336–1337. doi: 10.1111/head.13411
- Lipton RB, Cr Stock EG, Stock DA, et al. Rimegepant, an oral calcitonin gene-related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381(2):142–149.
- National Institute for Health and Care Excellence. Committee papers – Rimegepant for treating acute migraine [ID1539]. 2023 [cited 2023 Oct 23]. https://www.nice.org.uk/guidance/ta919/documents/committee-papers-3.
- Yu S, Ma L, Zhong Q, et al. Interim results of a long-term safety study of 75 mg rimegepant administered as needed in acute treatment of migraine among Chinese adults. Seoul, Republic of Korea: International Headache Congress; 2023.
- Gillard PJ, Devine B, Varon SF, et al. Mapping from disease-specific measures to health-state utility values in individuals with migraine. Value Health. 2012;15(3):485–494. doi: 10.1016/j.jval.2011.12.007.
- National Institute for Health and Clinical Excellence. Galcanezumab for preventing migraine: Technology appraisal guidance (TA659). Manchester: NICE; 2020 [cited April 2022]. https://www.nice.org.uk/guidance/ta659.
- Stafford M, Hareendran A, Ng-Mak D, et al. EQ-5D™-derived utility values for different levels of migraine severity from a UK sample of migraineurs. Health Qual Life Outcomes. 2012;10(1):65. doi: 10.1186/1477-7525-10-65.
- Vo P, Fang J, Bilitou A, et al. Patients’ perspective on the burden of migraine in Europe: a cross-sectional analysis of survey data in France, Germany, Italy, Spain, and the United Kingdom. J Headache Pain. 2018;19(1):82. doi: 10.1186/s10194-018-0907-6.
- British National Formulary. Drug acquisition cost for rimegepant 8-pack. 2022 [cited 2022 May 1]. https://bnf.nice.org.uk/drug/.
- Lipton R, Berman G, Kudrow D, et al. Long-term, open-label safety study of rimegepant 75 mg for the treatment of migraine (study 201): interim analysis of safety and exploratory efficacy (abstract P235LB). Headache. 2019;59(S1):175.
- Jones K, Burns A. Unit costs of health and social care Canterbury (kent). Personal Social Services Research Unit, University of Kent; 2021. https://kar.kent.ac.uk/92342/
- NHS Improvement. National schedule of NHS costs – Year 2019–2020. 2022. https://www.england.nhs.uk/national-cost-collection/.
- Schürks M, Rist P, Shapiro R, et al. Migraine and mortality: a systematic review and meta-analysis. Cephalalgia. 2011;31(12):1301–1314. doi: 10.1177/0333102411415879.
- Johnston K, Harris L, Powell L, et al. Monthly migraine days, tablet utilization, and quality of life associated with rimegepant – post hoc results from an open label safety study (BHV3000–201). J Headache Pain. 2022;23(1):10. doi: 10.1186/s10194-021-01378-5.
- Hubig LT, Smith T, Williams E, et al. Measuring interictal burden among people affected by migraine: a descriptive survey study. J Headache Pain. 2022;23(1):97. doi: 10.1186/s10194-022-01467-z.
- Lo SH, Gallop K, Smith T, et al. Real-World experience of interictal burden and treatment in migraine: a qualitative interview study. J Headache Pain. 202 2;23(1):65. doi: 10.1186/s10194-022-01429-5.
- Xu R, Insinga RP, Golden W, et al. EuroQol (EQ-5D) health utility scores for patients with migraine. Qual Life Res. 2011;20(4):601–608. doi: 10.1007/s11136-010-9783-5.
- Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programmes. Oxford: Oxford university press; 2015.
- Biohaven Pharmaceuticals Inc. Data on File: clinical study report BHV3000-201: a multicenter, open-label long-term safety study of BHV-3000 in the acute treatment of migraine. 2020.
- L’Italien G, Harris LA, Mohajer A, et al. Reduction in point prevalence of medication overuse headache following initiation of Nurtec ODT treatment– A real world administrative claims study. American Academy of Neurology 2022 Annual Meeting; April 2-7; Seattle, WA2022. doi: 10.1212/WNL.98.18_supplement.3698.
- Sacco S, Lampl C, Amin FM, et al. European headache federation (EHF) consensus on the definition of effective treatment of a migraine attack and of triptan failure. J Headache Pain. 2022 2022;23(1):133. doi: 10.1186/s10194-022-01502-z.
- Steiner TJ, Jensen R, Katsarava Z, et al. Aids to management of headache disorders in primary care (2nd edition): on behalf of the european headache federation and lifting the burden: the global campaign against headache. J Headache Pain. 2019;20(1):57. doi: 10.1186/s10194-018-0899-2.
- Turner IM, Pavlovic JM, B L R, et al. P0275: Patient preference, satisfaction, and improved clinical global impression of change with rimegepant 75 mg for the acute treatment of migraine: results from a long-term Open-Label safety study. The International Headache Congress – IHS and EHF Joint Congress 2021. J Headache Pain. 2021;22(1):103. doi: 10.1186/s10194-021-01293-9.