?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To estimate the cost-effectiveness of a treatment-pathway initiated with bimekizumab, a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)-17F and IL-17A, in patients with axial spondyloarthritis (axSpA) compared with IL-17Ai's, ixekizumab, and secukinumab, from the NHS Scotland perspective.
Methods
The axSpA treatment-pathway was modeled using a decision tree followed by a lifetime Markov model. The pathway included first- and second-line biologic disease-modifying antirheumatic drugs (bDMARD), followed by best supportive care (bDMARD, nonbiologic). Bimekizumab followed by any bDMARD (“BKZ”) was compared with IL-17Ai’s: secukinumab 150 mg followed by a blend (“SEC”) of dose up-titration to secukinumab 300 mg and any bDMARD, or ixekizumab followed by any bDMARD (“IXE”). Transition to the next therapy was triggered by Bath Ankylosing Spondylitis Disease Activity Index-50% (BASDAI50) non-response or any-cause discontinuation. A published network meta-analysis provided efficacy data. EuroQoL-5-dimensions utilities were derived by mapping from Ankylosing Spondylitis Disease Activity Score. Costs included disease management (linked to functional limitations), biologics acquisition (list prices), administration and monitoring (NHS 2021/22). Discounting was 3.5%/year. Probabilistic results from patients with non-radiographic axSpA and ankylosing spondylitis were averaged to reflect the axSpA disease spectrum. Scenario and sensitivity analyses were performed.
Results
The incremental cost-effectiveness ratio (ICER) of BKZ was £24,801/quality-adjusted life-year (QALY) vs. SEC (95% credible interval £24,163–£25,895). BKZ had similar costs (Δ -£385 [−£15,239–£14,468]) and QALYs (Δ 0.039 [−0.748–0.825]) to IXE, with £1,523 (£862–£2,222) net monetary benefit. Conclusions remained unchanged in most scenarios. Results’ drivers included BASDAI50 response rate and disease management cost.
Limitations
Results were based on list prices. Data concerning up-titration to secukinumab 300 mg was scarce.
Conclusions
The bimekizumab treatment-pathway represents a cost-effective option across the axSpA disease spectrum in Scotland. Bimekizumab is cost-effective compared to a secukinumab-pathway that includes dose up-titration, and has similar costs and QALYs to an ixekizumab-pathway.
Introduction
Axial spondyloarthritis (axSpA) is a chronic, immune-mediated inflammatory disease of the spine and sacroiliac joints (SIJ) that affects about 1.0% of the adult population worldwideCitation1 and 220,000 adults in the United Kingdom (UK)Citation2. AxSpA cases are evenly distributed between non-radiographic (nr-axSpA) and radiographic (r-axSpA), also known as ankylosing spondylitis (AS)Citation2–4. These two conditions constitute two subtypes of the same disease spectrumCitation5,Citation6. Patients with either condition suffer from the same range of debilitating symptoms including chronic back pain, pronounced stiffness, fatigue, peripheral manifestations such as enthesitis, peripheral arthritis and dactylitisCitation7,Citation8, as well as extra-articular manifestations such as anterior uveitis, psoriasis and inflammatory bowel diseaseCitation9. In addition to these clinical features, the diagnosis of nr-axSpA is also based on signs of inflammation frequently seen on magnetic resonance imaging (MRI) while a r-axSpA diagnosis requires visible damage to SIJ on conventional radiographs (x-ray)Citation7,Citation10. This distinction has been emphasized as relevant in clinical study settings, as most phase 3 trials of biologic disease-modifying antirheumatic drugs (bDMARDs) were indication-specificCitation11. In daily clinical practice, however, studies show that patients affected by nr-axSpA and r-axSpA experience a comparable burden of illness, with similar response to treatmentCitation12–15. For this reason, the 2022 guidelines for the management and treatment of axSpA developed by the Assessment of SpondyloArthritis international Society (ASAS) and the European Alliance of Associations for Rheumatology (EULAR) formulated recommendations seamlessly for both indicationsCitation16. One of these recommendations is to follow a treatment-pathway based on clinical response: patients with persistently high disease activity after a four-week treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) and physiotherapy are considered “non-responders,” and become eligible for a first-line biologic or targeted synthetic (b/ts) DMARD, with either a tumor necrosis factor-alpha inhibitor (TNFi), an interleukin-17 inhibitor (IL-17i) or a Janus kinase inhibitor (JAKi). Current practice suggests to first select a TNFi or an IL-17iCitation16. In case of first-line failure, as defined by an improvement <1.1 point of the Ankylosing Spondylitis Disease Activity Score (ASDAS) together with rheumatologist’s opinion, a switch to another b/tsDMARD is recommended. The guidelines do not specify any maximum number of treatment lines, nor further treatment pathway beyond the second-line, but instead refer to the overarching principle of “best care,” which is based on a shared decision between the patient and the rheumatologistCitation16.
Nevertheless, and despite several drug classes being available, clinical experts and patients with axSpA call attention to the high unmet clinical need for new treatmentsCitation17–19. Many patients with axSpA have an inadequate response or will lose response over time and require adjustments to their treatmentCitation20. Others cannot tolerate current treatments. Bimekizumab (Bimzelx, UCB Pharma S.A.) is a monoclonal IgG1 antibody selectively inhibiting IL-17F in addition to IL-17A that received marketing authorization in the European Union (EU) and the UK as an effective treatment for active axSpA with favorable benefit/risk profileCitation21. Bimekizumab significantly, rapidly and safely improved efficacy outcomes compared to placebo in the phase 3 trials BE MOBILE 1 (NCT03928704) in nr-axSpA and BE MOBILE 2 (NCT03928743) in r-axSpA)Citation22. Bimekizumab is now reimbursed for the treatment of adults with active axSpA who have responded inadequately or are intolerant to NSAIDs in several countries including ScotlandCitation23.
A new treatment with a novel mode of action is promising for patients with active axSpA, yet it represents an additional variable in the complex cost equation for the health care payer. Lessons learned from the recent health technology assessment (HTA) of IL-17Ai’s in axSpA include the necessity to upgrade previous models to a treatment sequencing frameworkCitation19,Citation24–27. We estimated the cost-effectiveness of an axSpA treatment-pathway initiated with bimekizumab rather than an IL-17Ai, secukinumab and ixekizumab, from the National Health Service (NHS) of Scotland perspective.
Methods
Population
Adult patients with active nr-axSpA or r-axSpA and insufficient response or tolerance to NSAIDs were modeled separately, by applying the baseline characteristics from phase 3 trials of bimekizumab, BE MOBILE 1 and BE MOBILE 2Citation22, respectively (). Active disease was defined as having a baseline score of 4 or more on the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). Results were pooled into a combined axSpA population representing the full spectrum of the disease, assuming an equal repartition of both indicationsCitation28.
Table 1. Cost-effectiveness model design and settings.
Intervention, comparators and treatment-pathways
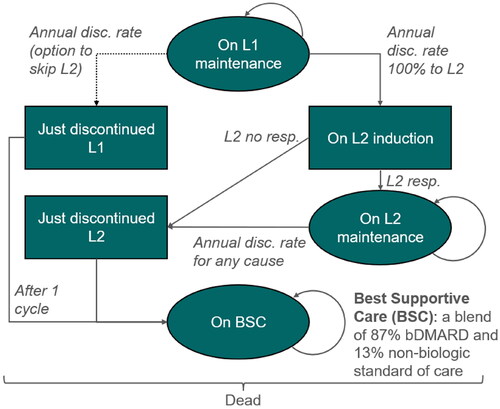
The intervention is the dual IL-17A/F inhibitor bimekizumab 160 mg administered subcutaneously (sc) every four weeks (Q4W)Citation29, which was compared to the IL-17Ai’s secukinumab 150 mg Q4W sc with 150 mg loading doses at weeks 0, 1, 2, 3 and 4 and dose up-titration to secukinumab 300 mg in case of inadequate responseCitation30, and ixekizumab 80 mg Q4W sc with 160 mg loading doseCitation31. Three different treatment-pathways were modeled to simulate sequential biologics starting with an IL-17i (). The bimekizumab-pathway (BKZ) consisted of a first-line with bimekizumab 160 mg followed by the TNFi class of drugs (as a “bDMARD standard of care”) in next line, and BSC in last line. The secukinumab-pathway (SEC) consisted of a first-line with secukinumab 150 mg followed by a blended next line of TNFi class or dose up-titration to secukinumab 300 mg in case of inadequate response, and BSC in last line. The blended next line was defined according to real-world data from the Rheumatic Diseases Portuguese Registry, reporting that 57% of patients initiating a treatment with secukinumab 150 mg for axSpA were up-titrated to secukinumab 300 mg in the maintenance phaseCitation32. The blended next line therefore assumed that 57% of patients received secukinumab 300 mg, and the remaining 43% received a TNFi. Finally, the ixekizumab-pathway (IXE) consisted of a first-line with ixekizumab 80 mg followed by the TNFi class in next line and BSC in last line.
Figure 1. AxSpA treatment-pathways applied in the model. AxSpA treatment-pathways applied in the model were defined in line with ASAS/EULAR and SmPC recommendations. In Line 2, TNFi were taken as a class, with efficacy data from an SLR/NMA and cost data based on individual TNFi list prices weighted by their estimated market shares in Scotland. ASAS, Assessment in Spondyloarthritis international Society; BSC, best supportive care; BKZ, bimekizumab treatment-pathway; b/ts, biologic/targeted synthetic; DMARD, disease modifying antirheumatic drug; EULAR, European League Against Rheumatism; IL, interleukin; IXE, ixekizumab treatment-pathway; nr-/r-axSpA, non-radiographic/radiographic axial spondyloarthritis; SEC, secukinumab treatment-pathway; SmPC, summary of product characteristics; TNFi, tumor necrosis factor-alpha inhibitor.

The “BSC” last line of treatment consisted of a blend of bDMARD standard of care (87% TNFi defined based on Scottish expert opinion; cf. Supplement S8), with the remaining 13% receiving non-biologic standard of care or no therapy. In the absence of ASAS/EULAR guidance beyond the second bDMARD, Scottish clinical and pharmacy experts were asked during an advisory board to describe later lines of therapy. Overall, they estimated that 87% of patients would still receive a bDMARD rather than receiving non-biologic conventional care: patients would either stay on their current treatment (13.7%), return to a previous effective treatment (58.8%), or receive the lowest cost bDMARD (14.7%). Real-world data also indicated that patients with an active disease would not revert to non-biologic conventional care after discontinuing a second-line biologicCitation33–35.
In the remainder of this article, bimekizumab, secukinumab and ixekizumab treatment-pathways are noted “BKZ,” “SEC,” and “IXE” respectively. The treatments’ names themselves are not abbreviated.
Outcomes and response definition
Clinical response was measured using the Bath Ankylosing Spondylitis Disease Activity Index 50% improvement (BASDAI50) in the base case analysisCitation36. The use of BASDAI50 is in line with Scottish clinical practiceCitation37. The Assessment in Spondyloarthritis international Society 40% improvement (ASAS40) was used as an alternative response criterion in a scenario analysisCitation38. Clinical scores estimated in the model included the Bath Ankylosing Spondylitis Functional Index (BASFI)Citation39, and the Axial Spondyloarthritis Disease Activity Score-C-reactive protein (ASDAS)Citation40. Both clinical response and change from baseline clinical score were measured 16 weeks after initiating an IL-17i, or 12 weeks after initiating a TNFi, as per prescription recommendations.
Data sources overview
Targeted literature reviews supported the model conceptualization and the collection of most recent health-related quality of life (HRQoL) and economic input parameters including unit costs and inflation indices from official webpages (NHS National Cost Collection, Personal Social Services Research Unit, British National Formulary [BNF])Citation41–43. A systematic literature review (SLR) and network meta-analysis (NMA) of randomized controlled trials published until January 2023Citation11 informed clinical efficacy inputs for the model. Clinical results stratified by responder status were obtained from the post-hoc analyses of the phase 3 trials of bimekizumab, BE MOBILE 1 in nr-axSpA and BE MOBILE 2 in r-axSpACitation22. Approved treatment schedules were retrieved from the summary of product characteristics (SmPC) of each comparator. Safety parameters were aligned with previously published technology appraisals of bDMARDs in axSpACitation19,Citation24. In addition to these sources, an advisory board, that included four Scottish rheumatologists and two prescribing pharmacists representing several NHS Scotland trusts was formed to bridge the data gaps and confirm that model assumptions and inputs were aligned with the NHS Scotland settings. This expert consultation also addressed questions on prescription patterns and treatment switching in patients with axSpA, helping define treatment-pathways in the model. Where necessary, broader UK data was assumed to apply to Scotland.
Model design
Our model was designed as a one-year decision tree (), followed by a lifetime Markov model with 12-week cycles (). Our model structure is similar to that of the model developed earlier by the University of York for an independent appraisal of TNFi cost-effectiveness in axSpA, National Institute for Health and Care Excellence (NICE) appraisal TA383Citation44. While alternative health economic models have been employed since TA383Citation45–48, the “York model” has been used as a base model and benchmark for several NICE and Scottish Medicines Consortium (SMC) cost-effectiveness submissions in axSpACitation19,Citation24–27,Citation49. Its original structure was thus retained as the most relevant for our purpose, but adapted to represent the axSpA treatment-pathway from first-line (L1) to second-line (L2) b/tsDMARD, followed by best supportive care (BSC) as a blend of biologic and non-biologic standard of careCitation16. Transition to the next line therapy was triggered by the absence of clinical response to the BASDAI50 after a 12-to-16 week assessment period, or upon any-cause discontinuation after having experienced an initial response. Compared to the York model, the time span of the decision tree was extended from 12 weeks to one year. This approach allowed to precisely calculate the treatment acquisition costs, by modeling the treatment-specific time to first response assessment, namely 16 weeks with IL-17i, and 12 weeks with TNFi. Additionally, the different pathways stratified by response status to first- and second-line were all captured, with their specific outcomes in the first year of treatment ().
Figure 2. Model structure: decision tree for first year treatment-pathway. Patients with active axSpA start a treatment-pathway with a first b/tsDMARD (L1). At the end of the initial 16-week assessment period, their clinical response is evaluated: (a) BASDAI50 responders continue initial treatment L1; a proportion of L1 responders will however discontinue L1 between w17 and w52 (any cause discontinuation, assumed to occur at an annual rate of 11%). After L1 discontinuation, patients get a next b/tsDMARD treatment (L2) which is assumed to start with the Markov model as from year 2. (b) BASDAI50 non responders stop initial treatment L1 and start a next b/tsDMARD treatment (L2) for another 16-week assessment period (or 12 weeks if L2 is a TNFi) with clinical response evaluation: BASDAI50 responders start L2 maintenance treatment, subject to a 11% annual discontinuation rate for any cause between w28 and w52, after which they receive BSC. BASDAI50 non-responders stop L2 treatment and receive BSC from w28 onwards until the end of the first year. The treatment-pathways modeled for one year determine the initial health states distribution for the Markov model. No discontinuation was assumed to occur during an assessment period (week 1–16 for L1, and week 17–28 for L2). axSpA, axial spondyloarthritis; BSC, best supportive care; L1/2, line 1/2; resp., response; Tx, treatment.
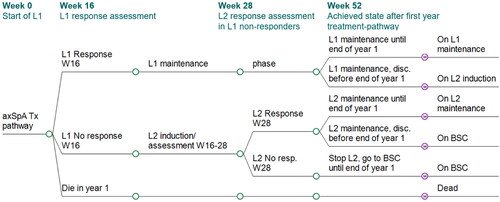
Figure 3. Model structure: Markov model as from second year of treatment until death. Initial distribution determined by outcome of decision tree. Three-month Markov cycles until end of horizon (or death). BSC, best supportive care; disc, discontinuation; L1/2, biologic line 1/2; resp, responder.
The health state distribution achieved at the end of the first year pathway determined the initial health state distribution in the Markov model (). The original 12-week Markov cycle length was preserved as it matches the time to first response assessment of the TNFi class of drugs, which forms the basis of second and third lines in our model. Mortality was modeled using life tables from the general UK population by age and sexCitation50. Having fitted a Gompertz-Makeham model to the mortality dataCitation51, a standardized mortality ratio (SMR) was applied (female 1.38, male 1.63) to account for a higher risk of death in axSpA patients compared to the general population, regardless of treatment and clinical responseCitation52. Events and transitions were adjusted to occur in the midpoint of a model cycle using the life-table method of within-cycle correctionCitation53. Lastly, an annual discount rate of 3.5% was applied to costs and benefits as recommended by the SMC. The model was programmed in Microsoft Excel. Key model settings and base case inputs values are presented in .
Efficacy and safety data
Efficacy at treatment level
Efficacy data were provided by an SLR and an NMA ()Citation11. Absolute clinical response rate and change from baseline (CfB) clinical scores at week 16 were obtained from bimekizumab phase 2b and phase 3 trials in axSpACitation22,Citation54. Relative efficacy of each comparator versus bimekizumab was applied via the relative risk (RR) of clinical response (BASDAI50, ASAS40), and the difference in mean CfB (DCfB) clinical scores (BASFI, ASDAS).
Table 2. Efficacy and safety data inputs.
Following response assessment at week 16, the clinical scores were stratified by responder status to establish “new baseline” scores for the next period, using a ratio between the mean CfB in responders and the mean CfB in non-responders, estimated from post-hoc analyses of BE MOBILE 1 and 2 trial data
In the next line, the treatment-specific NMA results were applied without any loss of response. The NMA was based on a population of predominantly bDMARD-naïve patients, thus the initial efficacy level was already influenced by the proportion of bDMARD experienced patients in the network (about 10%)Citation11. The redistribution of the treatment effect (CfB) following the second response assessment was done as described above. In the blended next line of secukinumab-pathway, a weighted average effect of treatments was calculated. TNFi efficacy was estimated in the NMA as a class effectCitation55, including adalimumab, certolizumab pegol, etanercept and golimumab sc; intravenous TNFi were not included, due to missing key endpointsCitation11. The dose up-titration to secukinumab 300 mg in inadequate responders was assumed to have the same efficacy as estimated for secukinumab 150 mg in the NMACitation56,Citation57; secukinumab 300 mg efficacy data were based on the unlicensed intravenous loading dose regimen, which was not included in the evidence network.
Clinical scores evolution in the first treatment year
Patients who experienced an initial clinical response at week 16 were further subject to an annual any-cause discontinuation rate of 11%Citation44. This value was aligned with discontinuation rates observed in week 16 responders of BE MOBILE 1 and 2 trials, between week 16 and 52 (post-hoc analyses). Assumptions concerning the evolution of clinical scores in the first-year of the treatment-pathway differed between the initial responders and non-responders, and by subsequent discontinuation status (supplement S2). Initial responders staying on first-line maintenance until the end of the year maintained the score achieved at week 16, whereas patients who discontinued before the end of the first year gradually and partially lost their initial gain. In case of discontinuation due to loss of effect, 50% of the initial CfB was assumed to be lost (by definition of lack of efficacy), else patients who discontinued due to other causes were assumed to lose 25% of their initial gain by the end of the first year.
Patients not responding to the first treatment line achieved the score of non-responders at week 16. Further score evolutions between week 16 and week 28 depended on the response to the second treatment line: the week 16 score was further improved by the CfB value stratified by second-line response status. Second-line responders maintained their week 28 score provided they did not discontinue, in which case they were assumed to gradually lose all previous gains by the end of the year. Non-responders to both first- and second-line were assumed to lose both initial and second CfB, and to return to the baseline score by the end of the first year.
Efficacy in last line
In the third and last line (“BSC”) of the treatment-pathway, patients on non-biologic standard of care experienced an annual worsening of BASFI score from 0.72% (nr-axSpA) to 1.57% (r-axSpA). These percentages were estimated via the mean BASFI change per 1 unit change in the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS)Citation58 multiplied by the mean annual change in mSASSSCitation59 and reported to the baseline BASFI score in BE MOBILE 1 and 2 trials. A generic effect of bDMARD, rather than a treatment-specific effect, was further applied as a slower rate of BASFI worsening (0.42, as calculated and applied in the York model for long-term efficacy) in the proportion of patients being on bDMARD standard of care in BSC (87%)Citation44.
Safety data
Two types of serious adverse events (SAEs) were captured in the model: tuberculosis reactivation, and other serious infections (including candidiasis and upper respiratory tract infections). The SAE rates were sourced from the BE MOBILE trials for bimekizumab, and from previous technology appraisals for other treatments ().
Health-related quality of life
Utilities for the model were derived from a mapping algorithm of the ASDAS instrument to the EQ-5D-3L with UK tariffs using a mixture model, i.e. a non-linear function of age, sex, and the current ASDAS score, which was implemented in the model to estimate utility scores over timeCitation60. This approach differs from the “York model” which predicted utility scores from a non-linear equation of BASFI and BASDAI scores. BASDAI is no longer the core efficacy outcome endorsed by ASAS-EULAR for treatment targetsCitation16. ASAS-EULAR now endorses the ASDAS efficacy measure for treatment targets, highlighting its improved performance in measuring disease activity. Because disease activity is an important determinant of HRQoL, the ASDAS-based utility mapping was more appropriate for this modelCitation61,Citation62. In the decision tree, the quality-adjusted life-years (QALYs) were estimated by summing up the areas under the curve (AUC) of utility scores per treatment phase using the trapezoidal ruleCitation63. In the Markov model, life-years spent in the different heath states were multiplied with the corresponding utility score to produce quality-adjusted life-years (QALYs).
Healthcare resource use and cost
Drug acquisition cost was calculated according to the licensed dose and schedule of each treatmentCitation29–31, with list prices sourced from the 2022 BNFCitation43. A weighted average of these prices was used to estimate the acquisition cost of TNFi as a class in second-line, as well as the acquisition cost of the bDMARD standard of care in BSC. Weights of individual TNFi were determined by their market shares in Scotland adjusted for TNFi usage in second-line or greater based on two combined sources (adalimumab 43%, etanercept 23%, infliximab 3%, certolizumab pegol 12%, golimumab 18%; Wilmington Scotland Market Share Data, October 2022 and RxY UK market share data, July 2022). From a cost perspective, using a TNFi mix including biosimilars is expected to be conservative by minimizing drug costs accrued in the long-term after discontinuation.
The type and frequency of healthcare resource use (HRU) required for drug administration (one training session with a nurse, followed by self-injection at no cost) and bDMARD routine monitoring (visits, imaging and laboratory tests) were aligned with the York model inputsCitation44. Unit costs of HRU were obtained from the NHS National Schedule of Reference Costs 2021-22Citation41, and the Unit costs of health and social care 2022Citation42. In addition to monitoring costs, annual disease management cost of axSpA including unplanned events (hospitalizations, visits, aids and appliances) were estimated using an established BASFI-based equationCitation44:
The original constant term (£1284.2) was inflated to 2022 prices using the NHS cost Inflation Index (NHSCII)Citation42. Health economic inputs of the model are summarized in .
Table 3. Healthcare resource use and cost inputs.
Analyses performed
Probabilistic analyses were run separately in the nr-axSpA and r-axSpA populations, each with 1,500 iterations. The probabilistic base case in the full axSpA disease spectrum was obtained by pooling the 3,000 iterations together, as several sources indicated a balanced repartition of nr- and r-axSpACitation2–4. The 3,000 iterations were used to derive 95% credible intervals (CrI) around the total, the incremental and the cost-effectiveness outcomesCitation64,Citation65. Seeding was used to ensure repeatable probabilistic results. Correlations between treatment effects for different comparators were accounted for in the probabilistic analysis by using the Convergence Diagnostic and Output Analysis (CODA) outputsCitation66.
In pairwise comparisons, total and incremental lifetime costs and QALYs with BKZ compared with alternative pathways (IXE, SEC) were presented, with the corresponding incremental cost-effectiveness ratio (ICER) and net monetary benefit (NMB) derived for BKZ vs. each pathway. A fully incremental analysis accounting for strong and extended dominance was also produced in line with SMC guidelines.
Univariate deterministic sensitivity analyses (DSA) were conducted using plausible ranges of values based on the lower and upper bounds of 95% credible intervals (CrI) or 95% confidence intervals (CI). The following scenario analyses were run: ASAS40 as an alternative response criterion to BASDAI50, assuming a 10%-point lower frequency of dose up-titration to secukinumab 300 mg (47% instead of 57%), TNFi in the next line replaced with JAKi (represented by upadacitinib, which has broad license and limited usage in axSpA in Scotland). A willingness-to-pay (WTP) threshold of £30,000 per QALY was used to evaluate cost-effectiveness.
Additional model outcomes obtained from the deterministic base case, including clinical score changes over time, retention rates and cost breakdown, were presented in supplement S5–S6.
Results
Cost-effectiveness
As compared to IL-17Ai-based pathways to treat patients with active axSpA, the bimekizumab-pathway generated largest amount of QALYs (mean 11.49, 95% CrI 11.47; 11.50) over a lifetime, for a mean total cost of £212,696 (95% CrI 211,985; 213,407). The ICER of BKZ compared to SEC was £24,801 with mean ΔQALY 0.590 (95% CrI −0.048; 1.227) and mean ΔCost £14,623 (95% CrI 5,829; 23,418). The mean (95% CrI) incremental NMB vs. SEC was £3,065 (2,453; 3,677) considering a WTP threshold of £30,000 per QALY gained (). BKZ had comparable costs and QALYs to IXE (mean ΔQALY 0.039 and 95% CrI −0.748; 0.825; mean ΔCost −£385 and 95% CrI −15,239; 14,468). No ICER was calculated vs. IXE given the numerically dominant position of BKZ vs. IXE. The mean incremental NMB vs. IXE was £1,542 (95% CrI 862; 2,222) at a WTP of £30,000 per QALY gained (). As seen on the multiple cost-effectiveness acceptability curve (CEAC), the probability of being cost-effective among the tested treatment-pathways increased in a comparable way for BKZ (from 21 to 37%) and IXE (from 29% to 38%) as WTP increased from £20,000 to £30,000 per QALY gained. In parallel, the probability decreased from 50% to 25% for SEC (). The “break-even” WTP value beyond which BKZ and IXE became more likely to be cost-effective than SEC occurred between £26,000 and £27,000 per QALY gained. The NMB of BKZ vs. IXE was positive (>0) thus considered cost-effective at WTP £30,000 per QALY, whereas the multiple CEAC at WTP £30,000 per QALY indicated a numerically higher probability of being cost-effective for IXE (38%) versus BKZ (37%). The former is based on average costs and QALYs, while the latter reflects the number of iterations maximizing the WTP, which caused an apparent discrepancy. Very small differences in NMA-based efficacy between bimekizumab and ixekizumab (), with high uncertainty due to limited data, made the comparison volatile. The cost-effectiveness planes (supplement S7) confirmed that BKZ and IXE could not be differentiated.
Figure 4. Multiple CEAC bimekizumab treatment-pathway vs. IL-17Ai treatment-pathways (axSpA population). BKZ and SEC cross at WTP £26,000 (probability of being cost-effective 33%). BKZ and IXE cross at WTP £31,000 (probability of being cost-effective 38%). Error bars indicate 95% confidence interval around the probability of being cost-effective. BKZ, bimekizumab treatment-pathway; CEAC, cost-effectiveness acceptability curve; IL, interleukin; IXE, ixekizumab treatment-pathway; nr-/r-axSpA, non-radiographic/radiographic axial spondyloarthritis; SEC, secukinumab treatment-pathway; WTP, willingness-to-pay.
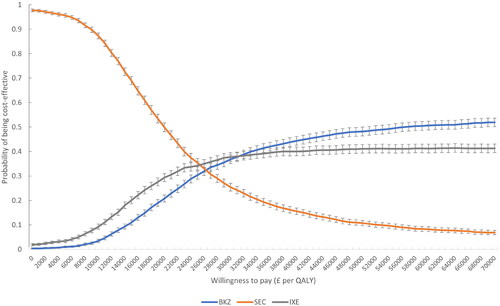
Table 4. Probabilistic base case, fully incremental cost-effectiveness results in axSpA population.
Table 5. Probabilistic base case, pairwise cost-effectiveness results in combined axSpA population.
Results specific for nr-axSpA and r-axSpA populations are presented in supplement S3.
Deterministic sensitivity analyses
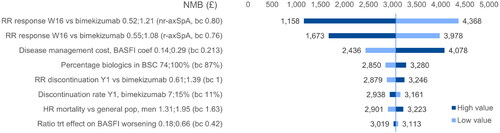
None of the tested ranges in the deterministic univariate sensitivity analyses led to a change in the base case conclusions. The three main results drivers were the relative risks of BASDAI50 response versus bimekizumab at week 16 in nr-axSpA and r-axSpA populations, and the value of the BASFI coefficient in the disease management cost equation. The percentage of biologics in the BSC blend was more specifically impacting the NMB of BKZ versus SEC (). Compared to IXE, the NMB was sensitive to the rate of discontinuation for any cause in the first treatment year, and to the relative risk of discontinuation versus bimekizumab ().
Figure 6. DSA BKZ vs. IXE (axSpA). NMB ranges obtained by applying the variations around the deterministic base case NMB to the probabilistic base case NMB. axSpA, axial spondyloarthritis; BASFI, Bath Ankylosing Spondylitis Functional Index; BKZ, bimekizumab treatment-pathway; NMB, net monetary benefit; nr, non-radiographic; trt, treatment; RR, relative risk.
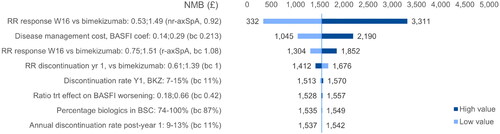
Scenario analyses
The outcomes of different scenarios explored did not change the overall conclusions of the base case analysis. As in the base case, IXE appeared numerically dominated by BKZ, but with comparable levels of costs and QALYs in each scenario. The ICER of BKZ vs. SEC ranged from £25,614 (using ASAS40 as response criterion) to £35,907 (JAKi-based second-line) ().
Table 6. Probabilistic scenarios analyses, axSpA population.
Clinical outcomes
Patients were aged 40 years at model entry and had a life expectancy of 36.4 years on average (20.6 discounted life-years). Within one year of treatment, the mean ASDAS score improved from a “very high” activity level of 3.7 to a “high” level with SEC (2.3) and to a “moderate” level with BKZ and IXE (2.0). The achieved ASDAS score of patients with initial response and on first-line maintenance at one year was 1.5–1.7 which is close to the “inactive disease” threshold (≤1.3). Since the initial benefit of first-line was integrated into a “new baseline” score upon second-line initiation and given that no loss of effect was assumed in subsequent lines, patients responding to second-line and remaining on second-line maintenance by the end of first year achieved a particularly low ASDAS score (0.5–0.8). Contrarily, patients who discontinued both first- and second-line within the first year returned to the baseline ASDAS “very high” level (3.7). The mean ASDAS score at five years was 2.4 with BKZ and IXE, and 2.7 with SEC. Similar trend was observed with the mean BASFI score over time: from 5.3 to 2.7 (BKZ, IXE) and 3.1 (SEC) at one year, and 3.5–3.8 at five years. First-line survival rates ranged from 31.2% (secukinumab 150 mg) to 40.0% (bimekizumab) at first year, and 19.9%–25.5% at five years, respectively. These percentages accounted for the stopping rule of initial non-responders and subsequent discontinuations for any cause.
Cost breakdown
Drug acquisition costs represented the largest component (50%–54%) of the total cost. The repartition of drug acquisition cost by line of therapy (Line 1/Line 2/BSC) was specific per treatment-pathway: 45%/17%/38% with BKZ and 44%/17%/39% with IXE, versus 22%/29%/49% with SEC. Disease management costs related to BASFI score represented the second largest cost item and were lowest with BKZ (£86,379) versus IXE (£87,885) and SEC (£88,965). Detailed cost breakdown is presented in supplement S6.
Discussion
Using a treatment-pathway approach, we aimed to anchor our cost-effectiveness analysis on the most recent axSpA treatment landscape and ASAS/EULAR recommendations. Efficacy inputs used in the model were informed by an SLR and NMA that covered a range of valid and recommended measures used to monitor axSpA in Scotland: initial response was assessed through the BASDAI50, the ASDAS score was connected to the estimation of utility scores rather than BASDAI and BASFI in previous cost-effectiveness studies, while BASFI score was used to estimate the disease management costs.
Our analysis using biologics list prices suggested that bimekizumab-pathway was cost-effective compared to a strategy based on the IL-17Ai secukinumab, at WTP £30,000 per QALY gained. The ICER compared to the secukinumab-pathway was £24,801 per QALY gained (95% CrI £23,972; 25,690). Compared to the ixekizumab-pathway, the lifetime costs and QALYs gained with the bimekizumab-pathway could not be differentiated based on large 95% credible intervals for incremental QALYs (−0.748; 0.825) and incremental cost (-£15,239; £14,468), and owing to limited efficacy data available for indirect comparison. Both IL-17A inhibitors were reviewed and recommended in axSpA by NICE with similar conditions of use, whereas the SMC declined ixekizumab reimbursementCitation67. The availability of bimekizumab, the first dual IL-17A and IL-17F inhibitor approved for active axSpA, would therefore represent a cost-effective alternative to address an unmet need in this population.
Previous models have demonstrated that secukinumab 150 mg was cost-effective in the UKCitation68 and FinlandCitation47 versus TNFi and conventional care, respectively. However, no sequences were planned in their base case, making the results difficult to compare to our model. Scenario analyses with a second-line bDMARD were tested by Purmonen et al.Citation47, however, these scenario analyses did not reflect prescribing patterns in routine clinical practice for secukinumab in axSpA because they omitted dose up-titration. Based on multiple data sources, including Scottish prescribers and international trends in real world evidence, use of secukinumab 300 mg in axSpA is common and increasing over timeCitation69–71, yet this pattern was not explored in previously published models. Recently, Le et al. investigated the cost-effectiveness of sequential use of bDMARD classes TNFi and IL-17Ai, using a patient-level simulation model borrowing most assumptions from the York modelCitation45. The best ICER was achieved with the sequence of a TNFi followed by an IL-17Ai, although none of the sequences were deemed cost-effective at a WTP of $100,000 per QALY gainedCitation45. The US context, with high drug cost prices, and a short time horizon of 10 years, limits the generalizability of the study results.
Strengths and limitations
Our cost-effectiveness model benefits from the solid basis of the York model which has been largely endorsed in previous submissions. The original model was upgraded with a novel approach to intervention and comparators, by modeling two consecutive lines of specific bDMARD treatments followed by BSC. The use of blended treatment lines allowed introducing features compatible with the 2022 ASAS-EULAR recommendations and local axSpA treatment guidelines: the dose up-titration to secukinumab 300 mg in about 60% of patients not adequately responding to secukinumab 150 mg, and a high percentage of bDMARD standard of care in BSC.
A pathway initiated with an IL-17i rather than a TNFi may not be common; it is a possibility as per ASAS-EULAR guidelines, however. Furthermore, the sequence was defined according to available phase 3 data for IL-17i, which hardly included TNFi-experienced patients. Efficacy data were therefore representative of a predominantly bDMARD-naïve population and were suitable to compare bimekizumab versus IL-17Ai, as first-line interventions.
The derivation of utility scores, thus QALYs, was also upgraded compared to previous axSpA models. The ASDAS mapping used UK tariffs for EQ-5D-3L, covered the full spectrum of axSpA, and was validated against cohorts recruited across Europe. The Alava algorithm was therefore an opportunity to replace previous unpublished, non-linear regressions of BASFI and BASDAI based on r-axSpA populations.
To the best of our knowledge, our analysis is also the first to present probabilistic base case results in a combined axSpA population.
The validity of a preliminary version of our model was reviewed by the York Health Economics Consortium. A pure non-biologic conventional care scenario in line with the “CC” arm of the York model was preserved in our model, populated with the placebo arm data of the NMA. This resulted in 9.50 QALYs and total cost of £117,400 which was consistent with the base case published by Corbett considering the cost inflation since 2016 (7.25/9.96 QALYs and £110,821/£89,500 for nr-axSpA/r-axSpA respectively)Citation44. Furthermore, the combination of a decision tree in the first year of treatment with a Markov model yielded clinical outcomes with good face validity. Important differences in terms of disease activity and functional impairments scores were predicted between patients staying on bDMARD (60%–70%) and those who experienced no initial response or early bDMARD discontinuation for any cause. The former group was close to the ASDAS “inactive disease” threshold, while the latter remained in a “high” activity state. Noteworthy, external validity of the modeled clinical outcomes was fair compared to data from the European Spondyloarthritis Research Collaboration Network (EuroSpA)Citation35. After one year, the mean ASDAS score in the SEC arm of the model dropped from 3.7 to 2.3, which is in line with the change from 3.6 to 2.5 reported by Michelsen et al.Citation35 for 1860 patients 12 months after secukinumab initiation. This analysis reported an average 12-month retention rate of 72% for secukinumab, ranging from 53% to 86% across the 13 participating registriesCitation35. In the model, an estimated 47% of patients on a secukinumab-pathway were still on either the 150 mg or the 300 mg dosage at one year. A direct comparison to EuroSpA data is not straightforward given the heterogeneity in observed retention rates, nevertheless, the modeled survival on secukinumab fell within the 95% confidence interval around the 53% retention estimated in the NOR-DMARD registry (43%–71%)Citation72. At a five-year horizon, the retention rate on either first- or second-line was 41%–49% across the three modeled treatment-pathways, which was lower than the 52%–65% drug survival rate after five years estimated in a UK registry of patients receiving TNFiCitation73, but consistent with the survival curves on TNFi from the DANBIO registryCitation74 and Swiss registryCitation75. In terms of life-years gained, our model using an axSpA-specific mortality (SMR 1.61) projected about 4 life-years lost compared to the general population (37 vs. 41 undiscounted life-years). This finding is supported by previous estimates in rheumatoid arthritisCitation76 and is more conservative than the 4.7–15.6 years of life lost reported for male-female (respectively) with r-axSpA and carriers of the HLA-B27 geneCitation77. Regardless of its face validity, life-years lost versus the general population should be considered as an illustrative outcome, because excess mortality was not linked to disease activity in the model, and an SMR may oversimplify the complex factors contributing to mortality, plus a simple half-cycle correction was applied in the first-year decision tree (owing to the rarity of death).
The results of our study should be interpreted with caution in context of few limitations. First of all, ICER values are based on list price while confidential discounts are in place, and subject to modifications over time. Another source of uncertainty linked to trial design should be noted. Contrary to real-practice, trials do not have a stopping rule at week 12/16, thus the stratification of outcomes by responder was done based on unpublished post-hoc evidence (ratio of scores changes in responders vs. non responders). Different ratios of change from baseline scores in responder vs. non-responders were generated earlier for HTA purposesCitation19,Citation24, which may allow undertaking a meta-analysis of post-hoc evidence. Moreover, the criteria used to assess clinical response and decide upon treatment continuation is not clear-cut. ASAS/EULAR have moved on from BASDAI50 to ASDAS scores, but Scotland continues to use BASDAI in clinical practice and the York model has broad historical use in axSpA. Upon treatment discontinuation, our assumption was a gradual loss of the initial gain -which yielded valid results at one-year- rather than an immediate rebound to baseline score as in York model. Neither of these assumptions are backed by solid evidence, however. Another critical point concerns the magnitude of a potential loss of effect in later treatment lines. Previously published studies based on the DANBIO registryCitation74 or clinical trial dataCitation49 suggested a sizeable loss of effect as the number of treatment lines increases. Another study highlighted a higher likelihood of response to stringent outcomes with the second TNFiCitation78. Our NMA did not highlight any specific pattern between the effects observed in bDMARD experienced versus naïve patientsCitation11. This might be explained by substantial missing outcome data in the experienced population, particularly in nr-axSpA. Similarly, estimating the impact of the treatment lines on drug retention rate remains uncertain. The issue with studies on retention rates is often the absence of clarity on whether primary non-responders are included or excluded in the statistics on discontinuationCitation24.
Last but important, the percentage of patients initiating secukinumab 150 mg and requiring a dose up-titration to 300 mg was not well documented at the time of model completion. In particular, no information on secukinumab dosage was available in the EuroSpA publicationsCitation35,Citation79. Our base case estimate of 57% was therefore sourced from a small (N = 32) registry study which had a suitable design to assess the frequency of dose escalationCitation32. Since then, a new registry study from Germany supported our base case estimate, as an up-titration to 300 mg during maintenance occurred in 46 patients out of 82 patients (56%) who initiated a treatment for axSpA with secukinumab 150 mgCitation70. Results of the expert consultation further suggested the predominance of secukinumab 300 mg dosage in ScotlandCitation70.
Conclusion
Based on list prices and the usual willingness-to-pay threshold, our cost-effectiveness analysis in patients with axSpA in Scotland showed that a treatment-pathway initiated with the IL-17A/F inhibitor bimekizumab was cost-effective compared to the secukinumab-pathway involving dose up-titration to 300 mg for inadequate responders to the 150 mg dose. The bimekizumab-pathway generated similar costs and QALYs compared to the ixekizumab-pathway. Results were robust to alternative assumptions and mostly sensitive to changes in the initial BASDAI50 response rates.
Transparency
Declaration of financial/other relationships
HT: Owns shares in Clifton Insight which has received consulting fees from UCB Pharma, Novartis, Pfizer, Roche, Eisai, Lundbeck, Argenx, Amicus, Merck, Daiichi-Sankyo, and Janssen. DW, MFM, MR, NL, VT: Employees of UCB Pharma. ML: independent consultant with no conflicts of interest to disclose. LG: Employee of IQVIA. Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author contributions
MM early model concept; VT: analyzed the data to provide model inputs; LG: searched literature, drafted the paper, programmed the model and ran the analyses; ML: model concept and inputs validation; NL: Late-stage analyses and developments; MR: model concept, analysis, interpretation, writing; HT: concept and analyses validation. All co-authors reviewed and contributed to the content.
Supplemental Material
Download MS Word (542 KB)Acknowledgements
The authors would like to thank the Scottish experts for their participation to the advisory board. The authors also thank Celia Menckeberg, UCB Pharma (The Netherlands) for publication coordination and editorial assistance, and Saurabh Trikha for his medical writing assistance during the preparation of this manuscript.
Additional information
Funding
References
- Reveille JD, Witter JP, Weisman MH. Prevalence of axial spondylarthritis in the United States: estimates from a cross-sectional survey. Arthritis Care Res. 2012;64(6):905–910. doi: 10.1002/acr.21621.
- National Axial Spondyloarthritis Society. Axial SpA (AS) facts and figures [Internet]. 2020 [cited 2020 May 31]. Available from: https://nass.co.uk/about-as/as-facts-and-figures/
- Akkoc N, Khan MA. Is axial spondyloarthritis more common than rheumatoid arthritis? Curr Rheumatol Rep. 2020;22(9):54. doi: 10.1007/s11926-020-00934-3.
- Strand V, Singh JA. Patient burden of axial spondyloarthritis. J Clin Rheumatol. 2017;23(7):383–391. doi: 10.1097/RHU.0000000000000589.
- Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390(10089):73–84. doi: 10.1016/S0140-6736(16)31591-4.
- Deodhar A, Strand V, Kay J, et al. The term “non-radiographic axial spondyloarthritis” is much more important to classify than to diagnose patients with axial spondyloarthritis. Ann Rheum Dis. 2016;75(5):791–794. doi: 10.1136/annrheumdis-2015-208852.
- Poddubnyy D. Challenges in non-radiographic axial spondyloarthritis. Joint Bone Spine. 2023;90(1):105468. doi: 10.1016/j.jbspin.2022.105468.
- de Winter JJ, van Mens LJ, van der Heijde D, et al. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther. 2016;18(1):196. doi: 10.1186/s13075-016-1093-z.
- Redeker I, Callhoff J, Hoffmann F, et al. The prevalence and impact of comorbidities on patients with axial spondyloarthritis: results from a nationwide population-based study. Arthritis Res Ther. 2020;22(1):210. doi: 10.1186/s13075-020-02301-0.
- Mease P, Deodhar A. Differentiating nonradiographic axial spondyloarthritis from its mimics: a narrative review. BMC Musculoskelet Disord. 2022;23(1):240. doi: 10.1186/s12891-022-05073-7.
- Deodhar A, Machado PM, Mørup M, et al. Comparative efficacy and safety of bimekizumab in axial spondyloarthritis: a systematic literature review and network meta-analysis. Rheumatology. 2023;kead598. doi: 10.1093/rheumatology/kead598.
- Hunter T, Sandoval D, Booth N, et al. Comparing symptoms, treatment patterns, and quality of life of ankylosing spondylitis and non-radiographic axial spondyloarthritis patients in the USA: findings from a patient and rheumatologist survey. Clin Rheumatol. 2021;40(8):3161–3167. doi: 10.1007/s10067-021-05642-6.
- Sieper J, Hu X, Black CM, et al. Systematic review of clinical, humanistic, and economic outcome comparisons between radiographic and non-radiographic axial spondyloarthritis. Semin Arthritis Rheum. 2017;46(6):746–753. doi: 10.1016/j.semarthrit.2016.09.002.
- Boonen A, Sieper J, van der Heijde D, et al. The burden of non-radiographic axial spondyloarthritis. Semin Arthritis Rheum. 2015;44(5):556–562. doi: 10.1016/j.semarthrit.2014.10.009.
- López-Medina C, Ramiro S, Heijde D V D, et al. Characteristics and burden of disease in patients with radiographic and non-radiographic axial spondyloarthritis: a comparison by systematic literature review and meta-analysis. RMD Open. 2019;5(2):e001108. doi: 10.1136/rmdopen-2019-001108.
- Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2022;82(9):e206. doi: 10.1136/ard-2023-223937.
- Winthrop KL, Isaacs JD, Mease PJ, et al. Unmet need in rheumatology: reports from the advances in targeted therapies meeting, 2022. Ann Rheum Dis. 2023;82(5):594–598. doi: 10.1136/ard-2022-223528.
- Kiltz U, Hoeper K, Hammel L, et al. Work participation in patients with axial spondyloarthritis: high prevalence of negative workplace experiences and long-term work impairment. RMD Open. 2023;9(1):e002663. doi: 10.1136/rmdopen-2022-002663.
- NICE TA718. TA718 | Ixekizumab for treating axial spondyloarthritis | Guidance | NICE [Internet]. NICE; 2021 [cited 2022 Jan 12]. Available from: https://www.nice.org.uk/guidance/ta718
- Navarro-Compán V, Ermann J, Poddubnyy D. A glance into the future of diagnosis and treatment of spondyloarthritis. Ther Adv Musculoskelet Dis. 2022;14:1759720X221111611. doi: 10.1177/1759720X221111611.
- Medicines and Healthcare Products Regulatory Agency. MHRA products | Bimzelx [Internet]. 2023 [cited 2023 Nov 16]. Available from: https://products.mhra.gov.uk/product/?product=BIMZELX
- Baraliakos X, Deodhar A, van der Heijde D, et al. Bimekizumab treatment in patients with active axial spondyloarthritis: 52-week efficacy and safety from the randomised parallel phase 3 BE MOBILE 1 and BE MOBILE 2 studies. Ann Rheum Dis. 2024;83(2):199–213. doi: 10.1136/ard-2023-224803.
- Scottish Medicines Consortium. Bimekizumab (Bimzelx) SMC2616 [Internet]. Scottish Medicines Consortium; 2023 [cited 2024 Feb 26]. Available from: https://www.scottishmedicines.org.uk/medicines-advice/bimekizumab-bimzelx-axspa-abb-smc2616/
- NICE TA719. TA719 | Secukinumab for treating non-radiographic axial spondyloarthritis | guidance [Internet]. NICE; 2021 [cited 2022 Jan 12]. Available from: https://www.nice.org.uk/guidance/ta719
- Scottish Medicines Consortium. Ixekizumab (Taltz), SMC2440 [Internet]. Scottish Medicines Consortium; 2022 [cited 2023 Nov 14]. Available from: https://www.scottishmedicines.org.uk/medicines-advice/ixekizumab-taltz-full-smc2440/
- Scottish Medicines Consortium. Secukinumab (Cosentyx), SMC2308 [Internet]. Scottish Medicines Consortium; 2021 [cited 2023 Nov 14]. Available from: https://www.scottishmedicines.org.uk/medicines-advice/secukinumab-cosentyx-full-smc2308/
- Scottish Medicines Consortium. Secukinumab (Cosentyx), ID1159/16 [Internet]. Scottish Medicines Consortium; 2016 [cited 2023 Nov 14]. Available from: https://www.scottishmedicines.org.uk/medicines-advice/secukinumab-cosentyx-fullsubmission-115916/
- Deodhar A, Mease PJ, Reveille JD, et al. Frequency of axial spondyloarthritis diagnosis among patients seen by US rheumatologists for evaluation of chronic back pain. Arthritis Rheumatol. 2016;68(7):1669–1676. doi: 10.1002/art.39612.
- EMC Bimekizumab. Bimzelx 160 mg solution for injection in pre-filled pen - summary of product characteristics (SmPC) - (emc) [Internet]. 2023 [cited 2023 Aug 9]. Available from: https://www.medicines.org.uk/emc/product/12834/smpc
- EMC Secukinumab. Cosentyx 150 mg solution for injection in pre-filled pen - summary of product characteristics (SmPC) - (emc) [Internet]. 2023 [cited 2023 Aug 9]. Available from: https://www.medicines.org.uk/emc/product/3669
- EMC ixekizumab. Taltz 80 mg solution for injection in pre-filled pen - summary of product characteristics (SmPC) - (emc) [Internet]. 2023 [cited 2023 Aug 9]. Available from: https://www.medicines.org.uk/emc/product/8199/smpc
- Fonseca D, Pinheiro FO, Rato M, et al. AB0483 can we predict which patients with spondyloarthritis will need dose escalation of secukinumab to 300 mg monthly? Ann Rheum Dis. 2021;80(Suppl 1):1269–1269. doi: 10.1136/annrheumdis-2021-eular.4001.
- Molto A, López-Medina C, den BF, et al. Efficacy of a tight-control and treat-to-target strategy in axial spondyloarthritis: results of the open-label, pragmatic, cluster-randomised TICOSPA trial. Ann Rheum Dis. 2021;80(11):1436–1444; [cited 2021 Jul 13]. Available from: https://ard.bmj.com/content/early/2021/05/05/annrheumdis-2020-219585 doi: 10.1136/annrheumdis-2020-219585.
- Glintborg B, Loft AG, Omerovic E, et al. To switch or not to switch: results of a nationwide guideline of mandatory switching from originator to biosimilar etanercept. One-year treatment outcomes in 2061 patients with inflammatory arthritis from the DANBIO registry. Ann Rheum Dis. 2019;78(2):192–200. doi: 10.1136/annrheumdis-2018-213474.
- Michelsen B, Lindström U, Codreanu C, et al. Drug retention, inactive disease and response rates in 1860 patients with axial spondyloarthritis initiating secukinumab treatment: routine care data from 13 registries in the EuroSpA collaboration. RMD Open. 2020;6(3):e001280. doi: 10.1136/rmdopen-2020-001280.
- Braun J, Davis J, Dougados M, et al. First update of the international ASAS consensus statement for the use of anti-TNF agents in patients with ankylosing spondylitis. Ann Rheum Dis. 2006;65(3):316–320. doi: 10.1136/ard.2005.040758.
- Borse RH, Kachroo S, Brown C, et al. Cost-effectiveness analysis of golimumab in the treatment of non-radiographic axial spondyloarthritis in Scotland. Rheumatol Ther. 2018;5(1):57–73. doi: 10.1007/s40744-018-0108-4.
- Brandt J, Listing J, Sieper J, et al. Development and preselection of criteria for short term improvement after anti-TNF treatment in ankylosing spondylitis. Ann Rheum Dis. 2004;63(11):1438–1444. doi: 10.1136/ard.2003.016717.
- Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the bath ankylosing spondylitis functional index. J Rheumatol. 1994;21:2281–2285.
- Machado P, Landewé R, Lie E, et al. Ankylosing spondylitis disease activity score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis. 2011;70(1):47–53. doi: 10.1136/ard.2010.138594.
- National Health Service. NHS England » national cost collection for the NHS [Internet]; 2022 [cited 2023 Nov 16]. Available from: https://www.england.nhs.uk/costing-in-the-nhs/national-cost-collection/
- PSSRU. Unit costs of health and social care programme (2022 – 2027) | the new home for the unit costs of health and social care report [Internet]; 2022 [cited 2023 Aug 9]. Available from: https://www.pssru.ac.uk/unitcostsreport/
- British National Formulary. MedicinesComplete—log in [Internet]; 2022 [cited 2023 Aug 9]. Available from: https://www.medicinescomplete.com/log-in/#/browse/bnf
- Corbett M, Soares M, Jhuti G, et al. Tumour necrosis factor-α inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis: a systematic review and economic evaluation. Health Technol Assess. 2016;20(9):1–334, v–vi. doi: 10.3310/hta20090.
- Le QA, Kang JH, Lee S, et al. Cost-Effectiveness of treatment strategies with biologics in accordance with treatment guidelines for ankylosing spondylitis: a patient-level model. J Manag Care Spec Pharm. 2020;26(10):1219–1231. doi: 10.18553/jmcp.2020.26.10.1219.
- Goeree R, Chiva-Razavi S, Gunda P, et al. Cost-effectiveness analysis of secukinumab in ankylosing spondylitis from the Canadian perspective. J Med Econ. 2019;22(1):45–52. doi: 10.1080/13696998.2018.1539400.
- Purmonen T, Puolakka K, Mishra D, et al. Cost-effectiveness of secukinumab compared to other biologics in the treatment of ankylosing spondylitis in Finland. Clinicoecon Outcomes Res. 2019;11:159–168. doi: 10.2147/CEOR.S192235.
- Jamal M, Kuijper TM, Hazes J, et al. A trial-based economic evaluation of the CaFaSpA referral strategy for axial spondyloarthritis. Scand J Rheumatol. 2023;53(1):1–9. doi: 10.1080/03009742.2023.2243081.
- NICE. TA407 | Secukinumab for active ankylosing spondylitis after treatment with non-steroidal anti-inflammatory drugs or TNF-alpha inhibitors | guidance | NICE [Internet]. NICE; 2016 [cited 2022 Jan 12]. Available from: https://www.nice.org.uk/guidance/ta407
- Office for National Statistics. National life tables - life expectancy in the UK: 2018 to 2020 - Office for National Statistics [Internet]; 2020 [cited 2023 Nov 16]. Available from: https://www.ons.gov.uk/releases/nationallifetableslifeexpectancyintheuk2018to2020
- Golubev A. Does Makeham make sense? Biogerontology. 2004;5(3):159–167. doi: 10.1023/B:BGEN.0000031153.63563.58.
- Bakland G, Gran JT, Nossent JC. Increased mortality in ankylosing spondylitis is related to disease activity. Ann Rheum Dis. 2011;70(11):1921–1925. doi: 10.1136/ard.2011.151191.
- Elbasha EH, Chhatwal J. Myths and misconceptions of within-cycle correction: a guide for modelers and decision makers. PharmacoEconomics. 2016;34(1):13–22. doi: 10.1007/s40273-015-0337-0.
- Baraliakos X, Deodhar A, Dougados M, et al. Safety and efficacy of bimekizumab in patients with active ankylosing spondylitis: three-year results from a phase IIb randomized controlled trial and its Open-Label extension study. Arthritis Rheumatol. 2022;74(12):1943–1958. doi: 10.1002/art.42282.
- Stevens JW, Fletcher C, Downey G, et al. A review of methods for comparing treatments evaluated in studies that form disconnected networks of evidence. Res Synth Methods. 2018;9(2):148–162. doi: 10.1002/jrsm.1278.
- Deodhar A, Kivitz A, Magrey M, et al. Op0023 a randomized, Double-Blind trial comparing secukinumab 300 mg and 150 mg at week 52 in patients with ankylosing spondylitis who did not achieve inactive disease during an initial 16 weeks of open-label treatment with secukinumab 150 mg. Ann Rheum Dis. 2022;81(Suppl 1):16–17. doi: 10.1136/annrheumdis-2022-eular.209.
- Song GG, Lee YH. Comparative efficacy and safety of secukinumab 75 mg, 150 mg, and 300 mg in patients with active ankylosing spondylitis. Int J Clin Pharmacol Ther. 2021;59(9):610–617. doi: 10.5414/CP203927.
- Landewé R, Dougados M, Mielants H, et al. Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann Rheum Dis. 2009;68(6):863–867. doi: 10.1136/ard.2008.091793.
- Ramiro S, Stolwijk C, van Tubergen A, et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow-up of the OASIS study. Ann Rheum Dis. 2015;74(1):52–59. doi: 10.1136/annrheumdis-2013-204055.
- Alava MH, Wailoo A, Chrysanthou G, et al. Measuring quality of life of patients with axial spondyloarthritis for economic evaluation. RMD Open. 2022;8(1):e001955. doi: 10.1136/rmdopen-2021-001955.
- Machado P, Landewé R, Braun J, et al. A stratified model for health outcomes in ankylosing spondylitis. Ann Rheum Dis. 2011;70(10):1758–1764. doi: 10.1136/ard.2011.150037.
- Hirano F, van der Heijde D, van Gaalen FA, et al. Determinants of the patient global assessment of well-being in early axial spondyloarthritis: 5-year longitudinal data from the DESIR cohort. Rheumatology. 2021;60(1):316–321. doi: 10.1093/rheumatology/keaa353.
- Yeh S-T. Using trapezoidal rule for the area under a curve calculation. SUGI 27 Proceedings, Paper 229-27, April 2002.
- Hatswell AJ, Bullement A, Briggs A, et al. Probabilistic sensitivity analysis in cost-effectiveness models: determining model convergence in cohort models. Pharmacoeconomics. 2018;36(12):1421–1426. doi: 10.1007/s40273-018-0697-3.
- Thom H. Deterministic and probabilistic analysis of a simple Markov model: how different could they be? Appl Health Econ Health Policy. 2022;20(3):447–449. doi: 10.1007/s40258-021-00700-1.
- Dias S, Sutton AJ, Welton NJ, et al. Evidence synthesis for decision making 6: embedding evidence synthesis in probabilistic cost-effectiveness analysis. Med Decis Making. 2013;33:671–678.
- Scottish Medicines Consortium. Ixekizumab 80mg solution for injection in pre-filled syringe or pen (Taltz®). SMC2440, June 2022.
- Emery P, Van Keep M, Beard S, et al. Cost effectiveness of secukinumab for the treatment of active ankylosing spondylitis in the UK. Pharmacoeconomics. 2018;36(8):1015–1027. doi: 10.1007/s40273-018-0675-9.
- Dubreuil M, Walsh JA, Deodhar A, et al. Real-world use of biologic disease-modifying anti‑rheumatic drugs in US patients with ankylosing spondylitis: persistence, factors associated with discontinuation, and dosing patterns. ISPOR | International Society for Pharmacoeconomics and Outcomes Research [Internet]; 2023 [cited 2024 Feb 6]. Available from: https://www.ispor.org/heor-resources/presentations-database/presentation/intl2023-3668/125411
- Anjohrin S, Song J, Abé C, et al. Real-world usage of biologic disease-Modifying anti-Rheumatic drugs in patients with axial spondyloarthritis in Germany. Value Health [Internet]; 2023;26(12):S300; [cited 2023 Dec 18]. Available from: https://www.ispor.org/heor-resources/presentations-database/presentation/euro2023-3785/131124
- Sivera F, Núñez-Monje V, Campos-Fernández C, et al. Real-world experience with secukinumab in the entire axial spondyloarthritis spectrum. Front Med. 2023;10:1156557. doi: 10.3389/fmed.2023.1156557.
- Olsen IC, Haavardsholm EA, Moholt E, et al. Nor-DMARD data management: implementation of data capture from electronic health records. Clin Exp Rheumatol. 2014;32:S-158–S-162.
- Yahya F, Gaffney K, Hamilton L, et al. Tumour necrosis factor inhibitor survival and predictors of response in axial spondyloarthritis-findings from a United Kingdom cohort. Rheumatology. 2018;57(4):619–624. doi: 10.1093/rheumatology/kex457.
- Glintborg B, Østergaard M, Krogh NS, et al. Clinical response, drug survival and predictors thereof in 432 ankylosing spondylitis patients after switching tumour necrosis factor α inhibitor therapy: results from the Danish nationwide DANBIO registry. Ann Rheum Dis. 2013;72(7):1149–1155. doi: 10.1136/annrheumdis-2012-201933.
- Hebeisen M, Scherer A, Micheroli R, et al. Comparison of drug survival on adalimumab, etanercept, golimumab and infliximab in patients with axial spondyloarthritis. PLoS One. 2019;14(5):e0216746. doi: 10.1371/journal.pone.0216746.
- Lassere MN, Rappo J, Portek IJ, et al. How many life years are lost in patients with rheumatoid arthritis? Secular cause-specific and all-cause mortality in rheumatoid arthritis, and their predictors in a long-term Australian cohort study. Intern Med J. 2013;43(1):66–72. doi: 10.1111/j.1445-5994.2012.02727.x.
- Li Z, Khan MK, van der Linden SM, et al. HLA-B27, axial spondyloarthritis and survival. Ann Rheum Dis. 2023;82(12):1558–1567. doi: 10.1136/ard-2023-224434.
- Manica SR, Sepriano A, Pimentel-Santos F, et al. Effectiveness of switching between TNF inhibitors in patients with axial spondyloarthritis: is the reason to switch relevant? Arthritis Res Ther. 2020;22(1):195. doi: 10.1186/s13075-020-02288-8.
- Glintborg B, Lindström U, Giuseppe DD, et al. One-year treatment outcomes of secukinumab versus tumor necrosis factor inhibitors in spondyloarthritis: results from five Nordic biologic registries including more than 10,000 treatment courses. Arthritis Care Res. 2022;74(5):748–758. doi: 10.1002/acr.24523.
- Haroon N, Inman RD, Learch TJ, et al. The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2013;65(10):2645–2654. doi: 10.1002/art.3807023818109.
- Machado PM, Landewé R, Heijde D V D Ankylosing spondylitis disease activity score (ASDAS): 2018 update of the nomenclature for disease activity states. Ann Rheum Dis. 2018;77(10):1539–1540. doi: 10.1136/annrheumdis-2018-213184.
- Deodhar A, Blanco R, Dokoupilová E, et al. Improvement of signs and symptoms of nonradiographic axial spondyloarthritis in patients treated with secukinumab: primary results of a randomized, placebo-controlled phase III study. Arthritis Rheumatol. 2021;73(1):110–120. doi: 10.1002/art.41477.
- Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373(26):2534–2548. doi: 10.1056/NEJMoa1505066.
- Deodhar A, van der Heijde D, Gensler LS, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (Coast-X): a randomised, placebo-controlled trial. Lancet. 2020;395(10217):53–64. doi: 10.1016/S0140-6736(19)32971-X.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (Coast-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392(10163):2441–2451. doi: 10.1016/S0140-6736(18)31946-9.
- Kiltz U, Baraliakos X, Brandt-Jrgens J, et al. Evaluation of the nonsteroidal anti-inflammatory drug-sparing effect of secukinumab in patients with ankylosing spondylitis: multicenter, randomised, double-blind, phase IV study. ACR meeting abstracts [Internet]; 2021 [cited 2024 Jan 31]. Available from: https://acrabstracts.org/abstract/evaluation-of-the-nonsteroidal-anti-inflammatory-drug-sparing-effect-of-secukinumab-in-patients-with-ankylosing-spondylitis-multicenter-randomised-double-blind-phase-iv-study/
- Xue Y, Hu J, Liu D, et al. Efficacy and safety of ixekizumab in Chinese patients with radiographic axial spondyloarthritis: 16-week results from a phase 3 study. ACR meeting abstracts [Internet]; 2022 [cited 2024 Jan 31]. Available from: https://acrabstracts.org/abstract/efficacy-and-safety-of-ixekizumab-in-chinese-patients-with-radiographic-axial-spondyloarthritis-16-week-results-from-a-phase-3-study/