Abstract
Objective
Attention-deficit/hyperactivity disorder (ADHD) medication is frequently associated with adverse events (AEs), but limited real-world data exist regarding their costs from a payer’s perspective. Therefore, this study evaluated the healthcare costs associated with common AEs among adult patients treated for ADHD in the US.
Methods
Eligible adults treated for ADHD were identified from a large US claims database (1 October 2015–30 September 2021). A retrospective cohort study design was used to assess excess healthcare costs and costs directly related to AE-specific claims per-patient-per-month (PPPM) associated with 10 selected AEs during ADHD treatment. To account for all costs associated with the AE, treatment episodes with a given AE were compared to similar treatment episodes without this AE. Entropy balancing was used to create cohorts with similar characteristics. Studied AEs were selected based on their prevalence in clinical trials for common ADHD medications and were identified from ICD-10-CM diagnosis codes recorded in claims.
Results
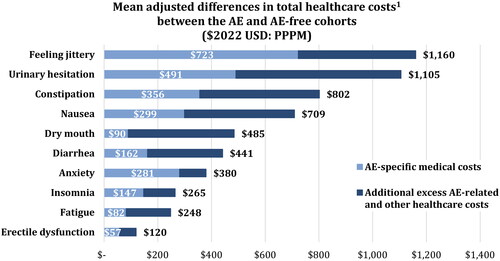
Among the 461,464 patients included (mean age: 34.2 years; 45.5% males), 49.4% had ≥1 AE during their treatment episode. Treatment episodes with AEs were associated with statistically significant AE-specific medical costs (erectile dysfunction: $57; fatigue: $82; dry mouth: $90; diarrhea: $162; insomnia: $147; anxiety: $281; nausea: $299; constipation: $356; urinary hesitation: $491; feeling jittery: $723) and excess healthcare costs PPPM (erectile dysfunction: $120, fatigue: $248, insomnia: $265, anxiety: $380, diarrhea: $441, dry mouth: $485, nausea: $709, constipation: $802, urinary hesitation: $1,105, feeling jittery: $1,160; p < .05).
Limitations
AEs were identified based on recorded diagnosis on medical claims and likely represent more severe AEs. Therefore, costs may not be representative of milder AEs.
Conclusions
This study found that AEs occurring during ADHD treatment episodes are associated with significant healthcare costs. This highlights the potential of treatments with favorable safety profiles to alleviate the burden experienced by patients and the healthcare system.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is characterized by a continuing pattern of inattention, hyperactivity, and impulsivity that hinders social, academic, and occupational functioning or developmentCitation1. Symptoms of ADHD often present during childhood; however, it can persist throughout a person’s lifetime, with an estimated overall prevalence in adults of 2.5%–4.4% and lifetime prevalence of 8.1% in the USCitation1–4.
Treatment for ADHD includes both pharmacologic and non-pharmacologic interventionsCitation5. Pharmacologic approaches remain the foundation of ADHD treatment and consist of short- and long-acting stimulant therapies, such as amphetamines or methylphenidates, and non-stimulant therapies (e.g. atomoxetine, clonidine, guanfacine)Citation5. Adverse events (AEs) are associated with both classes of medication and include insomnia, anxiety, nausea, fatigue, feeling jittery, urinary hesitation, constipation, dry mouth, diarrhea, and erectile dysfunctionCitation6,Citation7. AEs are a common cause of treatment modification, complicating management of ADHDCitation8,Citation9. Additional medical care resulting from AEs may lead to increased healthcare resource utilization and costsCitation9, thus contributing to the overall clinical and economic burden associated with ADHD. New treatments are presently in development that may be associated with an improved safety profile relative to currently approved treatmentsCitation7, potentially reducing the burden associated with ADHD in the US.
To date, studies have focused on the overall economic burden, treatment patterns, and medical expenditures associated with ADHD in adultsCitation9–14; however, little is known about the healthcare costs associated with AEs during treatment episodes in adults with ADHD. A recent matching-adjusted indirect comparison (MAIC) study provided insights regarding the comparative incidence of several AEs between centanafadine and Vyvanse (lisdexamfetamine dimesylate), Strattera (atomoxetine hydrochloride), and Qelbree (viloxazine extended release)Citation7; however, there is a lack of information on the economic burden associated with these AEs. A better understanding of the impact of AEs on patients with ADHD, and on the healthcare system, is needed to raise awareness among stakeholders regarding the importance of considering AEs in the management of ADHD, both from a clinical and healthcare policy perspective. Therefore, this study aims to assess healthcare costs associated with selected AEs among adult patients receiving treatment for ADHD in the US.
Methods
Data source
Claims data were obtained from the IQVIA PharMetrics Plus database from 1 October 2015 to 30 September 2021. This database contains fully adjudicated claims data from both medical and pharmacy plans. The data include information on demographics, enrollment, inpatient and outpatient diagnoses and procedures, inpatient stays, prescription fills, as well as the actual amount paid by health plans to the provider for services rendered. Data were de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act (HIPAA), therefore, institutional review board (IRB) approval was not necessary for this study per Title 45 of CFR, Part 46.101(b)(4) (https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/#46.101).
Study design
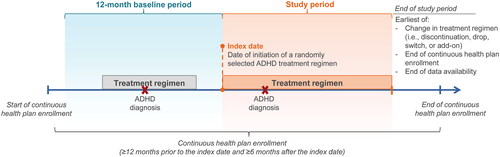
A retrospective cohort study design was used to assess the incremental healthcare costs associated with AEs during ADHD treatment by comparing treatment episodes with an AE to similar treatment episodes without the AE (). To capture a representative real-world sample of patients with varying disease duration and severity, the index date was defined as a randomly selected date on which a treatment regimen with one or more US Food and Drug Administration (FDA)-approved agents for the treatment of ADHD was newly initiated. Only initiation dates followed by at least six months of continuous health plan enrollment were eligible for selection as the index date. The index treatment (i.e. the treatment regimen initiated on the index date) was defined as all ADHD-related agents with a prescription fill within 30 days after the index date. The baseline period was defined as the 12-month period prior to the index date and served as a washout period. This time period was selected to (1) increase the likelihood of identifying new treatment initiation and (2) to allow for a sufficient observation period to assess patient characteristics, while keeping an adequately large sample size given that a longer observation period pre-index would lead to a smaller sample size. Lastly, the study period was the duration of a randomly selected treatment episode, defined as the period from the index date until the earliest of (i) a change in the treatment regimen (i.e. discontinuation, drop, switch, or add-on), (ii) the end of continuous health plan enrollment, or (iii) the end of data availability. Healthcare costs were assessed during the study period. There was no minimum duration required for the study period; however, patients were required to have continuous health plan enrollment during the baseline period and 6 months post-index to allow for a sufficient observation period post-treatment initiation. This was done to limit misclassification in a given cohort due to short follow-up post-treatment initiation as it may take some time before a patient meets with their physician and have their AE recorded, if any.
AEs included in this analysis were anxiety, constipation, diarrhea, dry mouth, erectile dysfunction, fatigue, feeling jittery, insomnia, nausea, and urinary hesitation. Selection of AEs was based on statistically significant risk difference derived from findings of a MAIC of lisdexamfetamine dimesylate, atomoxetine hydrochloride, and viloxazine extended release, and centanafadineCitation7. This MAIC analysis considered any AEs from each treatment’s clinical trial with at least 5% and twice the placebo rates in any of the treatment arms for a given comparisonCitation7,Citation15–18. Selected AEs were identified using ICD-10-CM diagnosis codes recorded in claims. Accordingly, lack of appetite was not considered despite being statistically significantly different across treatments in the MAIC analysis given that it could not be identified based on ICD-10-CM diagnosis codesCitation7.
Sample selection criteria and study cohorts
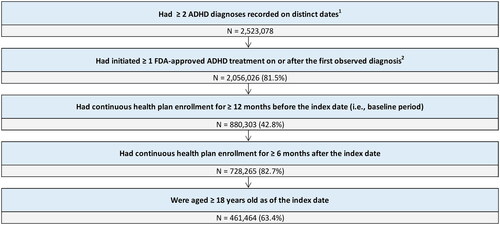
Adult patients (age ≥18 years) were required to have ≥2 ADHD diagnoses recorded on a medical claim on distinct dates, ≥1 prescription fill for an FDA-approved pharmacological treatment for ADHD on or after the first ADHD diagnosis, and continuous health plan enrollment for ≥12 months before the index date and for ≥6 months after the index date (). For each studied AE, eligible patients were classified into cohorts based on the presence of that AE. Patients in the AE cohort had no diagnosis for the given AE during the 12-month baseline period, but had ≥1 diagnosis for the given AE during the study period, increasing the likelihood of capturing AEs developed during a treatment episode. Patients in the AE-free cohort had no diagnoses for the given AE during both the baseline and study period. Given that the AE and AE-free cohorts were defined based on the presence of a given AE, patients were classified in the AE cohort for a given AE, and in the AE-free cohort for another AE. For example, if a patient had anxiety during the study period, this patient was classified as being in the AE cohort for anxiety but could also be classified as being in the AE-free cohort for another studied AE, such as nausea. Similarly, cohorts were not mutually exclusive across AEs.
Figure 2. Sample selection flow chart. ADHD, attention-deficit/hyperactivity disorder; FDA, Food and Drug Administration; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification. 1ADHD was defined as ICD-10-CM codes: F90.x. 2Patients could have initiated more than one treatment (i.e., multiple candidate index dates).
Measures, outcomes, and statistical analyses
Patient demographic and clinical characteristics were descriptively summarized, both before and after balancing for the AE and AE-free cohorts for each of the studied AEs, separately. Continuous variables were reported using means, medians, and standard deviations, while categorical variables were summarized using frequency counts and percentages. Costs were adjusted to 2022 USD using the medical component of the Consumer Price Index (CPI) and were reported from the payer’s perspective as the paid amount (i.e. the amount the payer paid the healthcare provider). Total excess healthcare costs included medical (i.e. inpatient, outpatient, emergency room visit, other) and pharmacy costs. AE-specific medical costs were defined based on a medical claim with a record of diagnosis for a given AE. As costs associated with AEs can exceed AE-specific medical costs, similar treatment episodes, with and without AEs, were compared to capture the full burden associated with a given AE. For instance, the costs associated with a diagnosis of fecal impaction would not be associated with constipation although it may result from severe or untreated constipation. These costs would be categorized under total excess, or incremental, healthcare costs. In addition, costs directly reported in claims with an AE-specific diagnosis were analyzed to provide further insights regarding the direct costs of managing the AE instead of the overall costs. Given that patients had different durations of follow-up, healthcare costs were reported as the average costs per-patient-per-month (PPPM).
Entropy balancing was used to balance key characteristics that may impact differences in outcomes across each of the AE and AE-free cohortsCitation19. Patients in the AE-free cohort were assigned a weight so that their specific covariate distribution had the same mean and standard deviation as the AE cohort for the following variables: age, gender, region, health plan type, calendar year, number of agents received between the ADHD diagnosis and the index date, Charlson Comorbidity Index (CCI score), specialist visits during the baseline period, and duration of index treatment regimen. A standardized difference of <0.2 between characteristics in each of the AE and AE-free cohorts was considered well balancedCitation20. Since the objective of the study was to assess the incremental burden of AEs in ADHD, comorbidities related to the given AE were not balanced as they may contribute to the disease burden.
Healthcare costs were compared between balanced (i.e. weighted) AE and AE-free cohorts using weighted two-part model generalized linear regression models (GLMs)—a method previously described in Wong et al.Citation21 Specifically, the first part estimated the odds of having positive costs for a given cost component (e.g. inpatient costs) using a weighted logistic regression model with a binomial distribution and the second part estimated positive costs using a weighted GLM with a log link and gamma distribution. For each studied AE, a separate regression was performed, where the dependent variable was the healthcare cost component, and the independent variable of interest was an indicator variable for having the given AE. The number of AEs, excluding the AE defining a given cohort, during the treatment episode was also included as covariate. p Values and 95% CIs were estimated using robust standard errors. Analyses were carried out using SAS Enterprise Guide statistical software program and Stata Statistical Software, Release 16.
Results
Patient characteristics
Among the patients who had an ADHD diagnosis (N = 2,523,078), a total of 461,464 met the eligibility criteria (). The average age of patients included in this study was 34.2 years, 45.5% were males, and 49.7% resided in the south (). In patients who had seen a specialist (29.4%), the mean number of visits was 1.5. The most common ADHD treatment initiated was stimulant monotherapy (95.0%) and 45.3% of patients were receiving first-line therapy. Nearly 1 in 2 patients (49.4%) had ≥1 medical claim for one of the 10 selected AEs during their treatment episode. The most prevalent AE was anxiety (37.0%), followed by fatigue (13.6%), insomnia (8.7%), nausea (5.9%), diarrhea (3.5%), constipation (3.0%), erectile dysfunction (1.7%), urinary hesitation (0.6%), feeling jittery (0.3%), and dry mouth (0.2%) (). It should be noted that this study was not designed to assess the prevalence of AEs and the reported values are based on diagnoses recorded in medical claims.
Figure 3. Prevalence of AEs during the study period. AE, adverse event. 1AEs were identified based on recorded diagnosis on a medical claim; prevalence was estimated from the proportion of patients with a diagnosis for a given AE recorded on a medical claim during their index treatment episode.
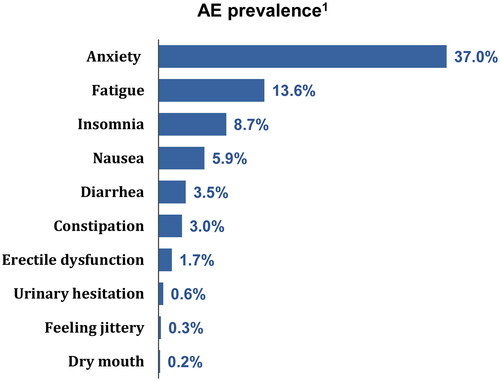
Table 1. Patient characteristics.
Study cohorts
Cohort sizes ranged between 823 and 170,573 patients. The most common AE was anxiety (170,573), followed by fatigue (62,569), insomnia (40,175), nausea (27,359), diarrhea (16,041), constipation (13,697), erectile dysfunction (7,848), urinary hesitation (2,623), feeling jittery (1,249), and dry mouth (823). Patient characteristics were largely consistent across individual AE cohorts (Supplemental Tables 1–10). Of note, most patients with urinary hesitation were males (60.2%). The number of specialist visits ranged from 1.5 to 3.7, with the most common reasons for visits being feeling jittery (3.7), dry mouth (2.8), and urinary hesitation (2.7).
Healthcare costs
All AEs were associated with statistically significantly (p < .05) increased healthcare costs PPPM, both overall and specifically for each AE (). Regarding overall costs, namely, the total excess medical and pharmacy costs associated with an AE, the least costly AE was erectile dysfunction ($120), followed by fatigue ($248), insomnia ($265), anxiety ($380), diarrhea ($441), dry mouth ($485), nausea ($709), constipation ($802), urinary hesitation ($1,105), and feeling jittery ($1,160). When considering costs directly related to claims for a diagnosed AE, the least costly AE was erectile dysfunction ($57), followed by fatigue ($82), dry mouth ($90), insomnia ($147), diarrhea ($162), anxiety ($281), nausea ($299), constipation ($356), urinary hesitation ($491), and feeling jittery ($723). As expected, the magnitude of AE-specific medical costs was reduced relative to overall healthcare costs; however, the order of costs was largely the same, with the exception of insomnia, anxiety, diarrhea, and dry mouth.
Discussion
This real-world retrospective cohort study evaluated the incremental healthcare costs associated with common AEs among adults with ADHD in the US. This study showed that AEs experienced during treatment for ADHD are associated with substantial healthcare costs, with the most costly AEs being feeling jittery, urinary hesitation, and constipation. Almost half of patients receiving treatment for ADHD experienced at least one AE, with the most common AEs being anxiety, fatigue, and insomnia. Incremental healthcare costs associated with AEs have the potential to generate considerable expenditures for payers when considering their corresponding prevalence. For example, the true burden of AEs can be better ascertained by multiplying the individual AE costs by the number of cases observed in this study and transposing the value into a hypothetical health plan of 1 million adults. In this scenario, the estimated annual incremental healthcare costs associated with AEs in ADHD would range from $0.4 million to $60.4 million, with anxiety ($60.4 million), nausea ($18.1 million), and fatigue ($14.5 million) accounting for the largest costs (). Taken together, these findings demonstrate the significant costs associated with AEs in ADHD and highlight how a reduction of AEs can lead to an important decrease in economic burden.
Figure 5. Estimated annual incremental healthcare costs associated with AEs per 1 million adults. AE, adverse event. 1Assuming 4.4% prevalence of ADHD (based on Kessler et al.Citation3); the proportion of treated patients and AE prevalence are based on findings from the current study. AE-specific medical costs were defined based on a medical claim with a recorded diagnosis for that given AE.
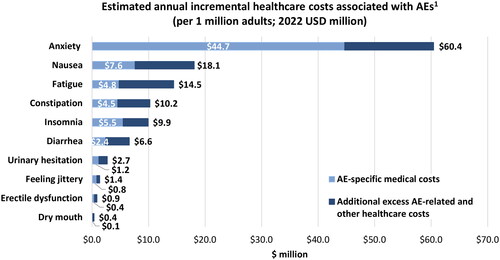
The costs of AEs are predominantly related to their prevalence. For instance, the AE-specific medical costs of anxiety in this study were $281 PPPM; however, given its high prevalence (37%), its annual costs were greater than AEs with a lower prevalence. Assuming each medical claim is associated to no more than one AE, a hypothetical plan of 1 million members would incur annual costs of approximately $72 million resulting from medical claims for the 10 AEs considered in this study among treated adults with ADHD (). This estimate is based on the proportion of treated patients with ADHD and AE prevalence from this study. A cost model that would allow the specification of ADHD prevalence, ADHD treatment, and AEs among treated patients for a given plan would be warranted, as it would allow health plans to identify their own costs with greater specificity.
Figure 6. Estimated annual AE-specific medical costs in a hypothetical health plan with 1 million members. ADHD, attention-deficit and hyperactivity disorder; AE, adverse event. 1AE prevalence was estimated from the proportion of patients with a diagnosis for a given AE recorded on a medical claim during their index treatment episode (based on total sample of 461,464 patients). An ADHD prevalence of 4.4% was assumed based on Kessler et al.3, and the proportion of treated patients was based on findings from the current study. 2Calculations are based on all AE-specific medical costs being independent – i.e. no more than one AE is recorded on an individual claim – and AEs lasting for the entire 12-month period.
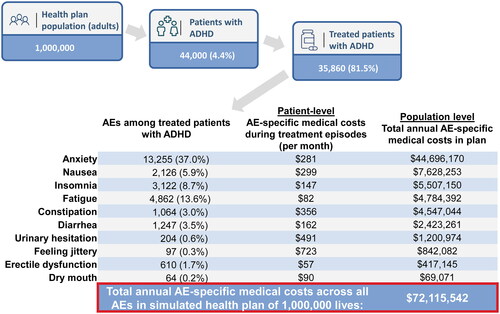
The AE-specific medical costs are likely to underestimate the costs of AEs in the management of ADHD, as they do not consider potential complications of these AEs. For example, only medical claims for which a diagnosis of constipation was recorded would be considered AE-specific medical costs, but costs associated with potential complications such as fecal impaction would not be considered. Nonetheless, AE-specific medical costs can lead to substantial annual costs if we consider the results of the current study within the context of the entire ADHD population in the US. Based on the findings of a study evaluating the economic burden of ADHDCitation13, AE-specific costs found in this study would account for approximately 38% of the costs of managing ADHD from a payers’ perspective. Although studies often report annual instead of monthly costs, as in this study, it should be emphasized that certain treatment-related AEs would not be expected to persist for an entire year. Therefore, annual costs may be less than what has been estimated in this example. Nevertheless, given that excess direct healthcare costs have been shown to account for 11.6% of the total economic burden of ADHDCitation13, reducing the impact of AEs would help lessen the overall burden of ADHD.
Prior studies have found that adult patients with ADHD experience a considerable clinical and economic burden, resulting from productivity and income losses, direct healthcare costs, and direct non-healthcare costs (e.g. disability, substance use disorder)Citation13,Citation22. While pharmacologic treatment for ADHD is beneficial and reduces annual medical costs compared with those not receiving treatmentCitation12, the rate of AEs is often high and requires careful monitoringCitation23. A recent study also found that patients who experience more AEs have a greater reduction in health-related quality of life, work productivity, and are more likely to skip planned doses to avoid AEsCitation24, which may lead to reduced treatment effectiveness. In both clinical and real-world studies, estimated rates of AEs for all types of ADHD medications ranged from 41%–96%Citation15,Citation16,Citation18,Citation24–26, with patients often experiencing several AEs simultaneouslyCitation24. In the present study, nearly 1 in 2 patients had at least 1 AE during their treatment episode, with an average of 1.4 AEs per patient, which aligns with the results of a chart review study evaluating reasons for treatment changes in adults with ADHDCitation26. Based on our findings, anxiety, fatigue, and insomnia were the most common AEs, occurring in 37%, 13.6%, and 8.7% of patients, respectively. Note that this study was not designed to evaluate the prevalence of the AEs and AEs included in this study were limited to those captured in a prior MAIC analysis. In addition, the estimates reported in this study correspond to the number of patients observed with recorded claims for the selected AEs, which does not take into account patients experiencing AEs who did not seek or receive formal medical care. As a result, the true prevalence and number of AEs experienced by patients with ADHD may be underestimated. For example, in a self-reported online survey among adults receiving medication for ADHD in the US, patients reported experiencing an average of 5.8 AEsCitation24, which is higher than what was reported in the current study (average of 1.4 out of 10 studied AEs). Furthermore, patients reported additional common AEs, such as depressed mood (45.6%), emotional impulsivity (45.1%), and headaches/migraines (39.0%), as well as a higher prevalence of studied AEs, notably, anxiety/panic attacks (47.7%), insomnia (47.9%), and fatigue (34.4%)Citation24. Therefore, the actual burden of AEs associated with treatment of ADHD is expected to be greater than what was observed in the current study, particularly when taking into the account the impact on health-related quality of life and work productivity. Collectively, findings from the literature suggest that improved management of ADHD may help to reduce the prevalence of AEs and the potential resulting reduction in treatment adherence.
All of the AEs evaluated in this study were associated with significantly increased total healthcare costs. Notably, important differences between the overall and AE-specific medical costs were observed, typically driven by inpatient costs associated with the sequelae of a given AE. Taking the example of a patient with severe, untreated constipation, the resulting fecal impaction may lead to emergency treatment and inpatient visits that are associated with significant costs. Therefore, the overall economic burden of the AE would extend beyond the costs of treating AE itself, as demonstrated in this study.
In addition to the costs observed during treatment episodes, prior studies have found that AEs can be an important driver of treatment modification, resulting in additional costs not captured in this studyCitation9,Citation26. For instance, a recent study evaluating treatment patterns among adult patients with ADHD found that treatment changes were frequent and occurred in approximately 50% of patientsCitation9. In that study, those who experienced treatment changes incurred an adjusted excess of $825 in annual healthcare costs compared with those who had no treatment changes, and increased with each additional treatment modificationCitation9. Another study revealed that AEs are cited as a reason for treatment modification in one in four patientsCitation26. Together, these studies further emphasize that the presence of AEs complicates ADHD treatment and contributes to its economic burden.
The high prevalence and significant economic impact of AEs associated with ADHD treatment observed in this study underscore the importance of physicians and patients considering the potential implications of possible AEs associated with various treatments when making treatment decisions and managing ADHD. For instance, among the AEs included in this study, anxiety had the highest prevalence and generated the largest associated costs from a payer’s perspective. Therefore, selecting treatments where anxiety is less common, for example, could help reducing associated healthcare costs. Given the availability of several pharmacologic treatments for ADHD and rising healthcare costsCitation5,Citation27, there is growing demand for evidence-based studies and economic models to inform healthcare and policy decisions more effectively—essential insights that this study provides. Furthermore, this study highlights the potential benefits of ADHD treatment with a favorable safety profile to alleviate the economic burden experienced by patients and the healthcare systemCitation7.
Limitations
The current study is subject to certain limitations inherent to retrospective databases using claims data, including the risk of data omission, coding errors, and the presence of rule-out diagnosis. As a result, patients may have been misclassified in a given study cohort. In addition, patients may have experienced AEs other than those selected in this study and may have had AEs for which they did not receive medical care, which would not be captured in this study. Moreover, it is not possible to determine whether AEs are treatment-related in this database. Although cohorts were balanced based on observable characteristics, there may have been residual confounding due to unobservable confounders (e.g. ADHD severity). Therefore, findings from this retrospective observational study should be interpreted as measures of association and no causal inference can be made. Given that this study only captures AEs recorded in claims data, it is likely that these AEs were more severe, and thus more costly than milder AEs that may not have been recorded in claims data. Consequently, this study may not be representative of less severe AEs, which may lead to an underestimation of the prevalence of AEs in this study. Lastly, the current study included only commercially insured patients, resulting in a sample that may not be representative of the entire US population with ADHD.
Conclusions
In this study, AEs occurring during ADHD treatment episodes were associated with significant healthcare costs both overall and specifically for studied AEs. This study also demonstrated that AEs requiring medical care are frequent during ADHD treatment episodes. As a result, these AEs lead to a considerable burden for patients and healthcare institutions and comprise a significant proportion of the total healthcare costs in the management of ADHD. Therefore, treatments for ADHD with a favorable safety profile may facilitate the effective management of ADHD both at the patient level (i.e. may lead to the reduction of treatment modification) and at the payer and healthcare institution level.
Transparency
Declaration of funding
This study was funded by Otsuka Pharmaceutical Development & Commercialization, Inc. The study sponsor was involved in several aspects of the research, including the study design, interpretation of data, writing of the manuscript, and decision to submit the manuscript for publication.
Declaration of financial/other relationships
JS is an employee of Otsuka Pharmaceutical Development & Commercialization, Inc. MC, RB, and MGL are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Otsuka Pharmaceutical Development & Commercialization, Inc. AC received research support from Allergan, Emalex, Akili, Cingulate, Corium, Lumos, Neurocentria, Otsuka, Purdue, Adlon, Sunovion, Tris, KemPharm, and Supernus; was on the advisory board of Corium, Otsuka, Tris, and Supernus; received consulting fees from Aytu, Cingulate, Corium, Lumos, Neurocentria, Noven, Otsuka, Tris, KemPharm, Supernus, and Tulex; received speaker fees from Takeda, Corium, Ironshore, Tris, and Supernus; and received writing support from Otsuka, Takeda, Corium, Ironshore, Purdue, and Tris. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors have made substantial contributions to the conception or design of the study, or the acquisition, analysis, or interpretation of data, drafting the manuscript and revising it critically for important intellectual content, and have provided final approval of this version to be published and agree to be accountable for all aspects of the work.
Ethics statement
Data were de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act (HIPAA) of 1996; therefore, no review by an institutional review board was required per Title 45 of CFR, Part 46.101(b)(4) (https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/#46.101).
Previous presentations
Part of the material in this manuscript was presented at the American Professional Society of ADHD and Related Disorders (APSARD) 2024 conference held 18–21 January 2024, in Orlando, FL as a poster presentation.
Supplemental Material
Download MS Word (89.9 KB)Acknowledgements
Medical writing assistance was provided by professional medical writer, Roxanne Wosu, MASc, an employee of Analysis Group, Inc., and was funded by Otsuka Pharmaceutical Development & Commercialization, Inc.
Data availability statement
The data that support the findings of this study are available from PharMetrics Plus, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Text Rev. Washington (DC): American Psychiatric Association; 2022.
- Faraone SV. Attention deficit hyperactivity disorder and premature death. Lancet. 2015;385(9983):2132–2133. doi: 10.1016/S0140-6736(14)61822-5.
- Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the national comorbidity survey replication. Am J Psychiatry. 2006;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716.
- Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593.
- Caye A, Swanson JM, Coghill D, et al. Treatment strategies for ADHD: an evidence-based guide to select optimal treatment. Mol Psychiatry. 2019;24(3):390–408. doi: 10.1038/s41380-018-0116-3.
- Post RE, Kurlansik SL. Diagnosis and management of adult attention-deficit/hyperactivity disorder. Am Fam Physician. 2012;85(9):890–896.
- Schein JC, Gauthier-Loiselle M, Catillon M, Childress A, editor Assessment of centanafadine in adults with ADHD: a matching adjusted indirect comparison vs lisdexamfetamine dimesylate, atomoxetine hydrochloride, and viloxazine extended release. Baltimore (MD): ASHP; 2023.
- Gajria K, Lu M, Sikirica V, et al. Adherence, persistence, and medication discontinuation in patients with attention-deficit/hyperactivity disorder - a systematic literature review. Neuropsychiatr Dis Treat. 2014;10:1543–1569.
- Schein J, Childress A, Adams J, et al. Treatment patterns among adults with attention-deficit/hyperactivity disorder in the United States: a retrospective claims study. Curr Med Res Opin. 2021;37(11):2007–2014. doi: 10.1080/03007995.2021.1968814.
- Adler LA, Farahbakhshian S, Romero B, et al. Healthcare provider perspectives on diagnosing and treating adults with attention-deficit/hyperactivity disorder. Postgrad Med. 2019;131(7):461–472. doi: 10.1080/00325481.2019.1647080.
- Daley D, Jacobsen RH, Lange AM, et al. The economic burden of adult attention deficit hyperactivity disorder: a sibling comparison cost analysis. Eur Psychiatry. 2019;61:41–48. doi: 10.1016/j.eurpsy.2019.06.011.
- Lee L, Arunajadai S, Mikl J, et al. The burden of attention-deficit/hyperactivity disorder in adults: a real-world linked data study. Prim Care Companion CNS Disord. 2023;25(2):22m03348. doi: 10.4088/PCC.22m03348.
- Schein J, Adler LA, Childress A, et al. Economic burden of attention-deficit/hyperactivity disorder among adults in the United States: a societal perspective. J Manag Care Spec Pharm. 2022;28(2):168–179. doi: 10.18553/jmcp.2021.21290.
- Witrick B, Zhang D, Su D, et al. Medical expenditures associated with Attention-Deficit/hyperactivity disorder among adults in the United States by age, 2015-2019. J Gen Intern Med. 2023;38(9):2082–2090. doi: 10.1007/s11606-023-08075-w.
- Adler LA, Adams J, Madera-McDonough J, et al. Efficacy, safety, and tolerability of centanafadine sustained-release tablets in adults with attention-deficit/hyperactivity disorder: results of 2 phase 3, randomized, double-blind, multicenter, placebo-controlled trials. J Clin Psychopharmacol. 2022;42(5):429–439. doi: 10.1097/JCP.0000000000001575.
- Adler LA, Goodman DW, Kollins SH, et al. Double-blind, placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69(9):1364–1373. doi: 10.4088/jcp.v69n0903.
- Adler LA, Spencer T, Brown TE, et al. Once-daily atomoxetine for adult attention-deficit/hyperactivity disorder: a 6-month, double-blind trial. J Clin Psychopharmacol. 2009;29(1):44–50. doi: 10.1097/JCP.0b013e318192e4a0.
- Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897–915. doi: 10.1007/s40263-022-00938-w.
- Hainmueller J. Entropy balancing for causal effects: a multivariate reweighting method to produce balanced samples in observational studies. Polit Anal. 2012;20(1):25–46. doi: 10.1093/pan/mpr025.
- Cohen J. Statistical power analysis for behavioural sciences. 2nd ed. New York, NY: Routledge; 1988.
- Wong W, Yim YM, Kim A, et al. Assessment of costs associated with adverse events in patients with cancer. PLoS One. 2018;13(4):e0196007. doi: 10.1371/journal.pone.0196007.
- Doshi JA, Hodgkins P, Kahle J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry. 2012;51(10):990 e2–1002 e2. doi: 10.1016/j.jaac.2012.07.008.
- Weibel S, Menard O, Ionita A, et al. Practical considerations for the evaluation and management of attention deficit hyperactivity disorder (ADHD) in adults. Encephale. 2020;46(1):30–40. doi: 10.1016/j.encep.2019.06.005.
- Schein J, Cloutier M, Gauthier-Loiselle M, et al. Symptoms associated with ADHD/treatment-related adverse side effects and their impact on quality of life and work productivity in adults with ADHD. Curr Med Res Opin. 2023;39(1):149–159. doi: 10.1080/03007995.2022.2122228.
- Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263–271. doi: 10.1055/a-1207-9851.
- Schein J, Childress A, Cloutier M, et al. Reasons for treatment changes in adults with attention-deficit/hyperactivity disorder: a chart review study. BMC Psychiatry. 2022;322(1):377. doi: 10.1186/s12888-022-04016-9.
- Keehan SP, Cuckler GA, Sisko AM, et al. National health expenditure projections, 2014-24: spending growth faster than recent trends. Health Aff. 2015;34(8):1407–1417. doi: 10.1377/hlthaff.2015.0600.