Abstract
Objective
To analyze the cost-effectiveness of transcatheter aortic valve implantation (TAVI) using the SAPIEN 3 (Edwards Lifesciences, Irvine, CA) compared to surgical aortic valve replacement (SAVR) in low- and intermediate-risk patients from a Japanese public healthcare payer perspective.
Methods
A Markov model cost-effectiveness analysis was developed. Clinical and utility data were extracted from a systematic literature review. Cost inputs were obtained from analysis of the Medical Data Vision claims database and supplemented with a targeted literature search. The robustness of the results was assessed using sensitivity analyses. Scenario analyses were performed to determine the impact of lower mean age (77.5 years) and the effect of two different long-term mortality hazard ratios (TAVI versus SAVR: 0.9–1.09) on both risk-level populations. This analysis was conducted according to the guidelines for cost-effectiveness evaluation in Japan from Core 2 Health.
Results
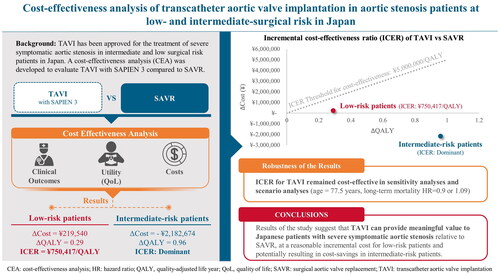
In intermediate-risk patients, TAVI was a dominant procedure (TAVI had lower cost and higher effectiveness). In low-risk patients, the incremental cost effectiveness ratio (ICER) for TAVI was ¥750,417/quality-adjusted-life-years (QALY), which was below the cost-effectiveness threshold of ¥5 million/QALY. The ICER for TAVI was robust to all tested sensitivity and scenario analyses.
Conclusions
TAVI was dominant and cost-effective compared to SAVR in intermediate- and low-risk patients, respectively. These results suggest that TAVI can provide meaningful value to Japanese patients relative to SAVR, at a reasonable incremental cost for patients at low surgical risk and potentially resulting in cost-savings in patients at intermediate surgical risk.
Graphical abstract
Cost-effectiveness analysis of transcatheter aortic valve implantation in aortic stenosis patients at low- and intermediate-surgical risk in Japan. Results demonstrate that TAVI can provide meaningful value to Japanese patients with severe symptomatic aortic stenosis relative to SAVR, at a reasonable incremental cost for low-risk patients and potentially resulting in cost-savings in intermediate-risk patients.
PLAIN LANGUAGE SUMMARY
Aortic Stenosis (AS) is the most common valvular heart disease in Japan, and, if left untreated, severe symptomatic AS (sSAS) is associated with a dramatic increase in mortality and morbidity. Transcatheter Aortic Valve Implantation (TAVI) is a minimally invasive treatment option for replacing the aortic valve in patients with sSAS and has been associated with similar or better outcomes compared to Surgical Aortic Valve Replacement (SAVR), which involves open-heart surgical replacement of the aortic valve. The objective of this study was to compare the costs and health outcomes associated with TAVI compared to SAVR in Japanese patients deemed low- or intermediate-risk for surgery. Despite the expanding use of TAVI in Japan, a cost-effectiveness analysis (CEA) does not exist that evaluates the economics of TAVI with the current generation SAPIEN 3 implant in patients with low- and intermediate-risk from a public perspective. Our study suggests that TAVI represents strong value for money among low- and intermediate-risk patients in Japan: compared to SAVR, TAVI is associated with better clinical outcomes and quality of life for patients, at a reasonable additional cost for low-risk patients and at a lower cost for intermediate-risk patients.
Introduction
Aortic stenosis (AS) is the most common type of valvular heart disease in high-income countries and its prevalence increases with age.Citation1,Citation2 If left untreated, severe symptomatic AS (ssAS) is associated with a dramatic increase in morbidity and mortalityCitation3,Citation4 and the progression of AS leads to increasing healthcare costs.Citation5 A recent study of valvular heart disease in Japan found that less than 10% of patients with AS underwent valve repair or replacement,Citation6 despite studies showing that valve replacement is associated with a substantial decrease in mortality and heart failure hospitalizations at 2 years in the Japanese population.Citation7
Transcatheter aortic valve implantation (TAVI) is an innovative treatment for ssAS, exhibiting short- and mid-term outcomes that are comparable or even superior to surgical aortic valve replacement (SAVR) regardless of surgical risk level.Citation8–12 The use of TAVI has dramatically increased in Japan since it was first performed in 2009, with a 740% increase in the number of TAVI cases from 2014 to 2019 and more than 170 Japanese centers performing TAVI in 2019.Citation13 Most recently, in March 2021, the Ministry of Health, Labor, and Welfare approved the treatment of patients with low surgical risk in Japan with SAPIEN 3 valves (Edwards Lifesciences, Irvine, CA) and gave reimbursement approval in August of the same year.Citation14 As such, it is expected that the utilization of TAVI may grow even further.
Given the rapidly aging population of Japan and the corresponding escalation of healthcare costs, the economic evaluation of new medical technologies – including transcatheter aortic valves – is increasingly important.Citation15 Despite the ever-expanding clinical implementation of TAVI in Japan, a cost-effectiveness analysis (CEA) does not exist that evaluates the economics of TAVI with the current generation SAPIEN 3 implant in patients with low and intermediate surgical risk from a public perspective. To address this gap in knowledge, we performed a CEA of TAVI with SAPIEN 3 in Japan in low- and intermediate-risk patients with ssAS from the perspective of the healthcare system in accordance with the guidelines for cost-effectiveness evaluation in Japan from Core 2 Health (C2H).Citation16
Methods
Study overview and population
A CEA was developed to evaluate two treatment options, namely TAVI with SAPIEN 3 compared to SAVR. To assess clinical benefit, the C2H guideline recommendations were followed and quality-adjusted life-years (QALYs) were utilized.Citation16 C2H is a department of the national institute of public health in Japan established to provide guidance for academic research on cost-effectiveness evaluation.Citation16 The analyses were conducted from a Japanese public healthcare payer perspective utilizing a lifetime horizon and applying a 2% discount rate to both costs and QALYs.Citation16 Populations of interest were ssAS patients at low and intermediate surgical risk, the definitions of which were based on indications of TAVI and risk levels described in published clinical trials.Citation8–10 Baseline patient age was set to an average age of 83 years as this is the average age of patients who underwent TAVI in Japan between 2019 and 2022, according to the Medical Data Vision (MDV) database. The MDV database is a large-scale Japanese administrative healthcare claims database derived from 480 hospitals accredited as providing acute care services in Japan, comprising approximately 26% of advanced treatment hospitals with information on the status and treatment of approximately 44 million patients.Citation17
Model framework
A literature-based cost-effectiveness model was employed to estimate direct healthcare costs and quality-adjusted survival for TAVI with SAPIEN 3 compared to SAVR. All patients started the model with procedure: either TAVI or SAVR, and early adverse events for TAVI or SAVR were captured in the procedure state using a decision tree. After 30 days, patients entered a Markov model and transitioned to the long-term health states. The Markov model was developed with specific transition probabilities for a given patient based on their selected treatment, with a monthly cycle for simulation of the first 5 years followed by 1-year cycles until all patients exited the model (lifetime horizon), commencing at the initial TAVI or SAVR procedure (). Transition between health states occurred through 1 year in the intermediate risk analysis and through 5 years in the low risk analysis, based on the duration of follow-up for each clinical study. Beyond the follow-up period, we made the conservative assumption that long-term clinical event rates were equivalent between TAVI and SAVR in the base case. In the base case, all-cause mortality after 1 year in the intermediate risk model and 5 years in the low-risk model was determined from the age-matched mortality of the general population in Japan.
Figure 1. Markov model based on complications observed in patients with ssAS. Abbreviations. PVL, Paravalvular leakage; SAVR, Surgical aortic valve replacement; ssAS, Severe Symptomatic Aortic Stenosis; TAVI, Transcatheter Aortic Valve Implantation.
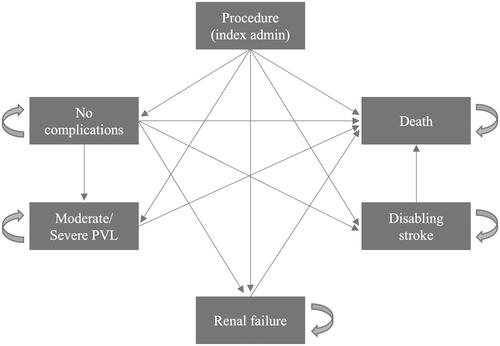
For the cost analysis, procedural complications were defined using International Classification of Disease (ICD)-10 codes and Kubun codes (Supplemental Table 1). The complication event rates were based on 30-day clinical outcomes from the PARTNER 3 and PARTNER 2 S3i trials.Citation9–12 The Markov model had the following post-treatment health states: dialysis, moderate/severe paravalvular leakage (PVL), disabling stroke, and death. Transition probabilities were derived from the PARTNER 2 S3i trial for intermediate-risk patients and the PARTNER 3 trial for low-risk patients. Patients exited the model by moving from any health state to death.
Model parameters
The clinical and utility outcomes for TAVI with SAPIEN 3 and SAVR were obtained through two systematic literature reviews (SLR) (see Supplemental Methods and Supplemental Figures 1 and 2 for details). For patients through 30 days post-treatment, the clinical outcomes were based on 30-day outcomes from low- and intermediate-risk patients from PARTNER 3 and PARTNER 2 S3i trials, respectively (). For the intermediate risk population, monthly transition probabilities for complications that occurred after 30 days were obtained from the 1-year data for PARTNER 2 S3i. Because 5 year data for the PARTNER 3 trial was recently published, the 5-year data for PARTNER 3 was used to obtain transition probabilities after 30 days in the low risk population.Citation23 The probability of death resulting from stroke, dialysis, and moderate/severe PVL as hazard ratios was derived from an analysis of the PARTNER 2 studies (Supplemental Table 2).Citation24 Age-based mortality rates after the clinical trial period (1 year for intermediate-risk and 5 years for low-risk) were obtained from Japan life tables (Supplemental Table 3).Citation25
Table 1. Clinical event probabilities and utilities used in the model.
The EQ-5D results from PARTNER 3 and PARTNER 2 S3i were used to determine the treatment utility weights (), converting to Japanese utilities using the value set from Shiroiwa et al.,Citation26 consistent with previous studies.Citation27,Citation28 For long-term disabling stroke and dialysis, the utility decrements for the Japanese population were obtained from a literature search similar to that done for clinical inputs (Supplemental Table 4). Moderate/severe PVL was not assumed to impact utility, since no utility decrements or values were available for patients with this health state.Citation21
Costs encompassed two categories: 1) Costs incurred in the hospital including device cost, procedure fee, and expenses associated with complications and 2) postoperative costs including long-term follow-up costs associated with disabling stroke and dialysis, as well as cost of rehospitalization ().
Table 2. Breakdown of TAVI and SAVR cost.
Implant and procedure costs were sourced from the medical fee information available from the Ministry of Health, Labor and Welfare (MHLW).Citation29,Citation30 These costs were obtained using revised reimbursement rates and adjusted to 2022.Citation35 The incremental costs of procedural complications were determined from analysis of MDV claims. Specifically, patients who received TAVI or SAVR with a diagnosis of AS between 2019 and 2022 were identified, and incremental expenses associated with complications were extracted and adjusted to the 2022 level. Cost of in-hospital hemorrhage/major bleeding was obtained from the literatureCitation31 and adjusted similarly. Data on long-term follow-up costs in the Japanese setting related to disabling stroke and dialysis and cost of hemorrhage occurring within hospitalization were not available through the MDV database, so these values were derived using a targeted literature search approachCitation32,Citation33 and adjusted to the most recent available year (2020) based on the medical care consumer price index tables.Citation36 Rehospitalization cost was obtained from an MDV-based analysis, in which hospitalizations with a heart failure diagnosis were identified, and the average cost for the inpatient stay was determined. Post-operative costs were assumed to be the same for patients who underwent TAVI with SAPIEN 3 and SAVR, consistent with previous work.Citation34 QALYs and costs were discounted at an annual rate of 2.0%, according to C2H guidelines.Citation16 Specific costs attributable to moderate/severe PVL were not available in MDV or from the literature.
Analytical methods
The cost-effectiveness of TAVI relative to SAVR for intermediate- and low-risk patient populations was determined by the incremental cost-effectiveness ratio (ICER). The ICER threshold for cost-effectiveness was set to ¥5 million/QALY, in accordance with the health technology assessment (HTA) system in Japan.Citation15,Citation37
A one-way deterministic sensitivity analysis (OWSA) was conducted to investigate the potential impact of uncertain parameters and assess the level of uncertainty in the outcomes of the model. Minimal and maximum values for one-way sensitivity analysis were obtained from the SLR for variables when available, otherwise predetermined upper and lower bounds were calculated for each parameter (Supplemental Table 5). A range of discount rates was also assessed. A complementary probabilistic sensitivity analysis (PSA) to establish robustness of the base case results was conducted using Monte Carlo simulation with 1000 iterations (Supplemental Table 6). The models were run using Excel with Visual Basic for Applications.
Two scenario analyses were conducted: one to test the model outcomes based on a younger average patient age, and the other to test different assumptions regarding the long-term risk of mortality between TAVI and SAVR. A younger patient population scenario analysis was conducted to examine the impact of a younger average patient age of 77.5 years, which is the midpoint of the age range of 75–80 years in which TAVI or SAVR are recommended by recent Japanese Circulation Society guidelines.Citation38 A second scenario analysis was conducted with two different assumptions to explore the possibility that long-term mortality could be different between individuals who underwent TAVI and those who underwent SAVR; it is prudent to explore the impact of different long-term mortality assumptions because only one publication has examined long-term mortality following SAPIEN 3 compared to SAVR.Citation39 Under the first assumption, long-term mortality beyond the trial follow-up was considered to be higher for TAVI than SAVR (hazard ratio [HR] = 1.09), based on PARTNER 2 trial five-year follow-up, which examined the earlier generation SAPIEN XT (Edwards Lifesciences, Irvine, CA) compared to SAVR.Citation40 Under the second assumption, long-term mortality beyond two years after treatment was considered to be lower for TAVI than SAVR (HR = 0.9). This was based on the PARTNER 2 S3i trial five-year follow-up, which considered SAPIEN 3 compared to SAVR.Citation41
Results
Base-case analysis
In the intermediate-risk population, TAVI resulted in a gain of 5.3 lifetime total QALYs, while SAVR resulted in a gain of 4.4 lifetime total QALYs (). The expected lifetime costs were ¥8.0 million for TAVI and ¥10.2 million for SAVR. As a result, the TAVI was a dominant procedure (TAVI had lower cost and higher effectiveness). In the low-risk population, TAVI led to a gain of lifetime total 9.0 life-years (LYs), while SAVR resulted in a gain of 8.6 lifetime total LYs. The expected lifetime costs were ¥6.7 million for TAVI and ¥6.5 million for SAVR. Hence, the ICER of TAVI compared to SAVR in patients with low risk level was ¥0.75 million/QALY. These ICER values were considerably lower than the predetermined cost-effectiveness threshold of ¥5 million/QALY gained.Citation15 Therefore, TAVI was found to be economically dominant in the intermediate-risk population and cost-effective in the low-risk population, when compared to SAVR.
Table 3. Base case analysis.
Sensitivity analysis
The OWSA allows for the investigation of the sensitivity of our model-based results to variations in specific parameters. The range of each parameter that is tested corresponds to a pre-specified range of possible values for that input. The tornado diagrams visually display that range of the ICER based on higher or lower values of each input, and this is ordered from widest variation (greatest impact on the outcome) to lowest variation in ICER. TAVI remained a dominant procedure in the intermediate-risk population after varying the input values for device cost (TAVI or SAVR), procedure costs, post-operative mortality, long-term cost of disabling stroke, and discount rate (). The intermediate-risk model was most sensitive to the cost of the TAVI implant.
Figure 2. Tornado diagrams of deterministic sensitivity analysis. A. Patients with intermediate surgical risk. B. Patients with low surgical risk. Abbreviations. QALY, Quality-Adjusted Life Year; SAVR, Surgical Aortic Valve Replacement; TAVI, Transcatheter Aortic Valve Implantation.
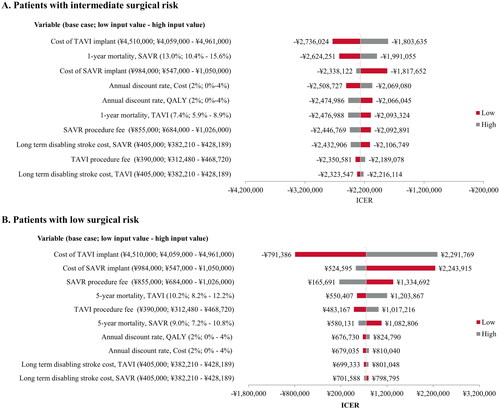
In the low-risk population, the ICER for TAVI versus SAVR remained cost-effective after varying the input values for device cost (TAVI or SAVR), procedure costs, post-operative mortality, long-term cost of stroke care, and discount rate (). The low-risk model was most sensitive to the cost of the implant, whether TAVI or SAVR. For the full deterministic sensitivity analysis results and breakdown of the costs incurred during hospitalization versus during follow-up, see Supplemental Tables 7–8 and Supplemental Figure 3.
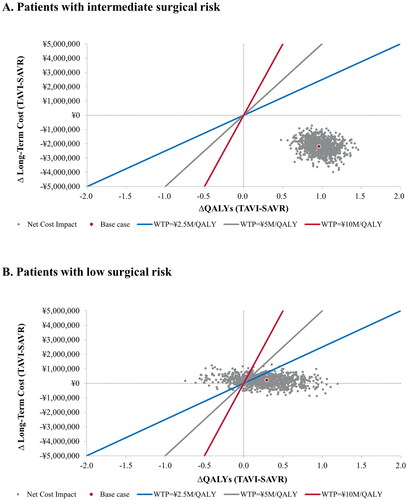
In the probabilistic sensitivity analysis, 100% of simulations for the intermediate-risk population were cost-effective at all willingness-to-pay (WTP) thresholds (). In the low-risk population, 86%, 92% and 96% of the simulations were cost effective at WTP thresholds of ¥2.5, 5 and 10 million/QALY, respectively (). These results indicate that TAVI is highly likely to be cost-effective compared to SAVR in both patient populations.
Scenario analyses
In the scenario analysis, in which starting age was adjusted from 83 years to 77.5 years, TAVI was a dominant (cost-saving) procedure in intermediate-risk patients, and cost-effective in low-risk patients (). In the scenario analysis designed to test the effect of varying long-term mortality benefits, TAVI remained dominant under both mortality risk assumptions for patients at intermediate surgical risk. Furthermore, TAVI remained cost-effective for low-risk patients under both mortality risk assumptions with ICERs of ¥1,989,801 and ¥435,484/QALY for mortality HR of 1.09 and 0.9, respectively ().
Table 4. Scenario analysis: Testing different patient starting age.
Table 5. Scenario analysis: Testing different long-term mortality benefits.
Discussion
This is the first study of its kind to examine the cost-effectiveness of TAVI using the most current SAPIEN 3 valve technology in Japanese patients at intermediate and low surgical risk, for whom TAVI was only recently approved. The results of this analysis demonstrated that TAVI was a cost-effective treatment option in intermediate- and low-risk patients with ssAS in Japan. With TAVI, reduced post-operative complications and non-procedure hospitalization costs (likely driven by differences in length of hospital stay) drove QALY gains and cost-savings, which greatly offset the device cost of SAPIEN 3 technology. The cost-effectiveness of TAVI persisted under two challenging scenario analyses, one examining a younger patient population and the second testing two different long-term mortality assumptions.
These CEA results are consistent with studies of low- and intermediate-risk patients conducted in EuropeCitation27,Citation43,Citation44 and North AmericaCitation45–47 that considered TAVI with SAPIEN 3. These previous studies report a wide range of results, depending on the perspective, patient risk level, and TAVI device generation considered. A recent review of TAVI cost effectiveness analyses found that TAVI was cost-effective in all analyses in low surgical risk patients, and TAVI was cost effective in all evaluations using the latest generation of balloon-expandable TAVI devices.Citation48 In this work, the SAPIEN 3 valve was prioritized as it represents a currently used technology and has been used in clinical practice in Japan for the last seven years.Citation49 With subsequent device generations and improved operator experience, the outcomes of TAVI will continue to improve, along with the economics.Citation50
The PARTNER 3 trial did not capture the planned revascularization strategy, so it was not possible to perform a subgroup analysis on addition of percutaneous coronary intervention (PCI). However, we performed a scenario analysis according to whether PCI or CABG was performed during the index hospitalization for TAVI and SAVR patients, respectively (Supplemental Table 9). Patients who underwent TAVI with PCI or SAVR with CABG had slightly higher lifetime total costs compared to those without PCI or CABG, respectively. This is consistent with findings from previous work.Citation51
Healthcare systems and perspectives vary between countries, making regionally-specific CEAs important to optimizing clinical practice and particularly important in the rapidly aging society of Japan. There are only two previous CEAs of TAVI versus SAVR in Japan, and the findings regarding the cost-effectiveness of TAVI across these studies are inconsistent.Citation34,Citation52 Of note, both studies relied on data using older generation SAPIEN valve implants and evaluated the cost-effectiveness of TAVI in higher risk populations. Inoue et al. (2020) concluded that TAVI with SAPIEN-XT was cost-effective versus SAVR and standard of care in high-risk and inoperable patients with an ICER of 1,337,525/QALY and 3,460,810/QALY, respectively.Citation34 Kodera et al. (2017) found that TAVI with the first-generation SAPIEN valve was cost-effective versus medical management in inoperable patients with an ICER of 3,918,808/QALY, but that TAVI with SAPIEN XT was not cost-effective versus SAVR in intermediate-risk patients.Citation52
There are a few reasons why our study may have found that TAVI was an economically dominant procedure in the intermediate risk population compared with SAVR. Firstly, the SAPIEN XT valve is an older, 2nd generation implant, whereas the SAPIEN 3 valve is the most current implant with a smaller profile (which allows for smaller vascular access) and a sealing skirt (which allows for reduced rates of PVL). Accordingly, it would be expected improved clinical outcomes and reduced cost related to the complications such as bleeding, vascular access issues and PVL when using the SAPIEN 3 implant. Additionally, the present work used MDV claims data specifically from individuals diagnosed with AS, whereas complication costs and follow-up costs for TAVI and SAVR used by Kodera et al. were based on a general cardiovascular population and may not reflect the real-world costs and complications associated with ssAS patients.
The present study also differs in its consideration of the impact of trial-reported postoperative complications on long-term costs based on published costs of care, while the study from Inoue et al. relies on expert opinion to account for a smaller scope of long-term costs. Lastly, Kodera et al. determined costs based on a simple accounting of the device and procedure cost and assumed that complication and follow up costs did not differ for TAVI and SAVR, whereas the present study employed trial data combined with claims data to account for non-procedure costs. Using claims data for costs is considered more robust for a cost analysis because it includes information on insurance payment as well as clinical diagnoses and procedure codes to ascertain medical conditions and treatments.Citation53 Taken together, the different costing methodology and assessment of TAVI using a newer generation device with likely better procedural outcomes could account for the discrepancy in findings between our study and that of prior CEAs.
Our sensitivity analyses indicated that cost-effectiveness of TAVI versus SAVR was somewhat sensitive to the cost of SAVR implant and post-SAVR complications. It is known that these costs may vary widely between hospitals. According to the MDV database, the median overall hospitalization cost of SAVR, excluding the device and procedure fee, was ¥3,347,549 with an interquartile range of ¥1,753,677. This is more substantial than the variation in the overall cost of TAVI, which had a median of ¥1,021,489 with an interquartile range of ¥858,383. This suggests that SAVR hospital economics may vary more widely than TAVI in Japanese hospitals (possibly driven by variation in outcomes). Therefore, cost-effectiveness results should be interpreted with the understanding that an institutions’ outcomes and economics may greatly influence the cost-effectiveness of TAVI versus SAVR at individual facilities.
Limitations
The results of this study should be interpreted in the context of several limitations. First, in the examination of intermediate-risk patients, no randomized controlled trial data that focused on TAVI using SAPIEN 3 vs. SAVR were available. That said, a propensity-matched data set derived from the SAVR arm of the PARTNER 2 A trial and the TAVI arm of the PARTNER S3i registry has been used in other analyses and so event rates were derived from this data.Citation12
Second, the model does not account for different costs or outcomes for reintervention between TAVI and SAVR in this patient group. Our decision to exclude reintervention costs in our CEA is consistent with other published modelsCitation34,Citation52, as there is little evidence directly comparing long-term rates of reintervention between SAVR and TAVI patients or the cost implications of failing bioprosthetic valves. Furthermore, current data from the United Kingdom Transcatheter Aortic Valve Implantation registry has showed excellent long-term transcatheter aortic valve function, with 91% of patients free of structural valve deterioration between 5 and 10 years post-implantation,Citation54 thereby suggesting that the difference between SAVR and TAVI implant durability is likely minimal. Since we would assume that valve reintervention would likely be reflected in mortality, the sensitivity analysis in which the mortality HR of SAVR vs. TAVI was varied should account for any substantial differences in reintervention rates between the two treatment strategies. The model also does not account for institutional variations such as the presence of a disciplined heart team, for two reasons: 1) TAVI is rapidly expanding in Japan and 2) a recent analysis showed that outcomes for patients undergoing TAVI in Japan are consistent across hospitals regardless of their annual procedural volume.Citation55
Third, the model assumed no utility impact of the moderate/severe PVL health state because no utility values were available in the literature, especially in Japanese patients. This assumption may need reexamination in future research, as one study found a small, non-significant quality of life decrement for patients with moderate/severe PVL based on the Kansas City Cardiomyopathy Questionnaire score, which cannot be used to determine utility.Citation24
Fourth, some recent cost-effectiveness analyses comparing TAVI and SAVR in low-risk patients have included atrial fibrillation as a health state.Citation43,Citation56 In the development of our model, we had to be parsimonious in the selection of health states relevant to the treatment and the Japanese healthcare setting. We relied on a targeted literature review of existing models as well as expert input specific to the Japanese treatment perspective in the selection of our health states. Our model accounts for the impact of atrial fibrillation by including the incremental cost of atrial fibrillation as a procedural complication in the first cycle of the model, but not as a long-term health state. The inclusion of an atrial fibrillation state in this model would likely increase the cost difference and QALY difference between SAVR and TAVI, given the higher rates of new onset atrial fibrillation in the SAVR arms of the PARTNER 2S3i and PARTNER 3 trials.Citation11,Citation12 Therefore, our study – which includes atrial fibrillation as a procedural complication rather than a health state – is a conservative estimate of the cost-effectiveness of TAVI.
Fifth, there are limitations inherent to claims database analyses, such as potential inaccuracies or omissions in the source records. Nevertheless, cost information derived from MDV claims data in this study provides important insights by facilitating meaningful cost-effectiveness comparisons in a large sample of Japanese patients with AS. Similarly, the MDV database did not allow stratification of peri-operative costs according to surgical risk level. However, in Japan SAVR has long been approved for all risk levels, whereas TAVI was approved only in high-risk patients until 2021. It is important to note that patients with higher risk incur higher index hospitalization costs.Citation57 Therefore, in the MDV data from 2019–2022 that were used to determine costs, it is reasonable to assume that there was a greater proportion of low-risk/low-cost patients undergoing SAVR than TAVI, potentially resulting in underestimation of the costs of SAVR compared to TAVI. As TAVI becomes used more broadly in low-risk patients, the average cost of TAVI across all patients can be expected to decrease, lessening the cost differential between TAVI and SAVR. Further, the scenario analyses showed that cost-effectiveness of TAVI with SAPIEN 3 remains robust across patient ages and long-term mortality assumptions.
Lastly, there are inherent limitations to cost effectiveness analyses, and assumptions are made based on the best available data; these assumptions may not account for all potential differences across patient populations and healthcare systems.
Conclusions
The present study is the first time that the cost-effectiveness of TAVI with SAPIEN 3 compared to SAVR has been studied in intermediate- and low-risk patients from the Japanese healthcare system perspective. TAVI was dominant in intermediate-risk patients and well below the cost-effectiveness threshold in low-risk patients and these results were stable across a variety of scenario analyses and sensitivity analyses. These results suggest that TAVI can provide meaningful value to Japanese patients at a reasonable incremental cost for patients with low surgical risk and potentially cost-saving in patients with intermediate surgical risk.
Transparency
Declaration of financial/other relationships
JK has no conflict of interest.
SB discloses the following: Abiomed (Consultant; Research Grant); Boston Scientific Corp (Advisory Board Member; Research Grant); Edwards Lifesciences (Speaking Honoraria); Medtronic (Speaking Honoraria; Advisory Board member); Shockwave (Advisory Board Member; Consultant); Zoll Medical (Advisory Board Member; Speaking Honoraria).
KT received lecture fees from Abbott Medical Japan Co., Ltd and Edwards Lifesciences, Medtronic, and Daiichi Sankyo Co., Ltd., and is a Proctor of Edwards Lifesciences and Medtronic.
CT, XJ, and KY disclose employment with Edwards Lifesciences.
Author contributions
JK, SB, and CT participated in study design. CT and XJ conducted the statistical analyses. All authors interpreted the results. JK, KT, and SB wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
Data availability and materials
Due to its proprietary nature, supporting data cannot be made openly available. All summary data relevant to these results are present within the report. Further information about the data and conditions for access are available from Medical Data Vision, Co. (https://en.mdv.co.jp).
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (555.1 KB)Acknowledgements
The authors thank Emily Farra. and Farah Pathan of Boston Strategic Partners, Inc. for editorial contributions and assistance with manuscript preparation, supported by Edwards Lifesciences.
Additional information
Funding
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011. doi: 10.1016/S0140-6736(06)69208-8.
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366(18):1696–1704. doi: 10.1056/NEJMoa1202277.
- Campo J, Tsoris A, Kruse J, et al. Prognosis of severe asymptomatic aortic stenosis with and without surgery. Ann Thorac Surg. 2019;108(1):74–79. doi: 10.1016/j.athoracsur.2019.01.031.
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of cardiology. American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e35–e71.
- Clark MA, Arnold SV, Duhay FG, et al. Five-year clinical and economic outcomes among patients with medically managed severe aortic stenosis: results from a medicare claims analysis. Circ Cardiovasc Qual Outcomes. 2012;5(5):697–704. doi: 10.1161/CIRCOUTCOMES.112.966002.
- Izumi C, Matsuyama R, Asaoka M, et al. Valvular heart disease in Japan: characteristics and treatment of patients in acute care hospitals in 2019. J Cardiol. 2023;82(1):29–34. doi: 10.1016/j.jjcc.2023.03.007.
- Takeji Y, Taniguchi T, Morimoto T, et al. Transcatheter aortic valve implantation versus conservative management for severe aortic stenosis in real clinical practice. PLOS One. 2019;14(9):e0222979. doi: 10.1371/journal.pone.0222979.
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198. doi: 10.1056/NEJMoa1103510.
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–1620. doi: 10.1056/NEJMoa1514616.
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–1705. doi: 10.1056/NEJMoa1814052.
- Leon MB, Mack MJ, Hahn RT, et al. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. 2021;77(9):1149–1161. doi: 10.1016/j.jacc.2020.12.052.
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387(10034):2218–2225. doi: 10.1016/S0140-6736(16)30073-3.
- Kaneko T, Vemulapalli S, Kohsaka S, et al. Practice patterns and outcomes of transcatheter aortic valve replacement in the United States and Japan: a report from joint data harmonization initiative of STS/ACC TVT and J‐TVT. J Am Heart Assoc. 2022;11(6):e023848. doi: 10.1161/JAHA.121.023848.
- Notice on the calculation of the price of medical materials for specific insurance. (no. 305009) 2020. Available from: https://www.mhlw.go.jp/web/t_doc?dataId=00tc4902&dataType=1&pageNo=2.
- Shiroiwa T, Fukuda T, Ikeda S, et al. Development of an official guideline for the economic evaluation of drugs/medical devices in Japan. Value Health. 2017;20(3):372–378. doi: 10.1016/j.jval.2016.08.726.
- Guideline for Preparing Cost-Effectiveness Evaluation to the Central Social Insurance Medical Council Ministry of Health, Labour, and Welfare. 2022 [13 April 2023]. Available from: https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf.
- MDV database overview: MDV EBM insight. [October 24, 2023]. Available from: https://en.mdv.co.jp/ebm/about-mdv-database/mdv-database-overview/.
- Baron SJ, Thourani VH, Kodali S, et al. Effect of SAPIEN 3 transcatheter valve implantation on health status in patients with severe aortic stenosis at intermediate surgical risk: results from the PARTNER S3i trial. JACC: cardiovascular Interventions. 2018;11(12):1188–1198.
- Shiroiwa T, Fukuda T, Ikeda S, et al. Japanese population norms for preference-based measures: EQ-5D-3L, EQ-5D-5L, and SF-6D. Qual Life Res. 2016;25(3):707–719. doi: 10.1007/s11136-015-1108-2.
- Baron SJ, Magnuson EA, Lu M, et al. Health status after transcatheter versus surgical aortic valve replacement in low-risk patients with aortic stenosis. J Am Coll Cardiol. 2019;74(23):2833–2842. doi: 10.1016/j.jacc.2019.09.007.
- Shiroiwa T, Noto S, Fukuda T. Japanese population norms of EQ-5D-5L and health utilities index mark 3: disutility catalog by disease and symptom in community settings. Value Health. 2021;24(8):1193–1202. doi: 10.1016/j.jval.2021.03.010.
- Shimizu U, Aoki H, Sakagami M, et al. Walking ability, anxiety and depression, significantly decrease EuroQol 5-Dimension 5-level scores in older hemodialysis patients in Japan. Arch Gerontol Geriatr. 2018;78:96–100. doi: 10.1016/j.archger.2018.06.006.
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement in low-risk patients at five years. N Engl J Med. 2023;389(21):1949–1960. doi: 10.1056/NEJMoa2307447.
- Arnold SV, Zhang Y, Baron SJ, et al. Impact of short-term complications on mortality and quality of life after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12(4):362–369. doi: 10.1016/j.jcin.2018.11.008.
- Ministry of Health LaW. Handbook of health and welfare statistics. 2021. [Online]. Available from: https://www.mhlw.go.jp/english/database/db-hh/1-2.html.
- Shiroiwa T, Murata T, Morii Y, et al. Comparison of value set based on DCE and/or TTO data: scoring for EQ-5D-5L health states in Japan. Health Qual Life Outcomes. 2016; 22(1):16–654. doi: 10.1016/j.jval.2016.03.1834.
- Kuck KH, Leidl R, Frankenstein L, et al. Cost-Effectiveness of SAPIEN 3 transcatheter aortic valve implantation versus surgical aortic valve replacement in german severe aortic stenosis patients at low surgical mortality risk. Adv Ther. 2023;40(3):1031–1046. doi: 10.1007/s12325-022-02392-y.
- Pinar E, García de Lara J, Hurtado J, et al. Cost-effectiveness analysis of the SAPIEN 3 transcatheter aortic valve implant in patients with symptomatic severe aortic stenosis. Rev Esp Cardiol. 2022;75(4):325–333. doi: 10.1016/j.rec.2021.02.013.
- Medical fee information provision service: transcatheter prosthesis valve set: Ministry of Health, Labour and Welfare. 2022 [21 Mar 2023]. Available from: https://shinryohoshu.mhlw.go.jp/shinryohoshu/searchMenu/doSearchInputTp.
- Medical fee information provision service: transcatheter valve replacement: Ministry of Health, Labour and Welfare. 2022 [21 March 2023]. Available from: https://shinryohoshu.mhlw.go.jp/shinryohoshu/searchMenu/doSearchInputSp.
- Kamae I, Hashimoto Y, Koretsune Y, et al. Cost-effectiveness analysis of apixaban against warfarin for stroke prevention in patients with nonvalvular atrial fibrillation in Japan. Clin Ther. 2015;37(12):2837–2851. doi: 10.1016/j.clinthera.2015.10.007.
- Hattori N, Hirayama T, Katayama Y. Medical care for chronic-phase stroke in Japan. Neurol Med Chir. 2012;52(4):175–180. doi: 10.2176/nmc.52.175.
- Cost of dialysis treatment: Cost burden when receiving dialysis. Available at. https://www.zjk.or.jp/kidney-disease/expense/dialysis/. [21 Mar 2023]. Available from: https://www.zjk.or.jp/kidney-disease/expense/dialysis/.
- Inoue S, Nakao K, Hanyu M, et al. Cost-effectiveness of transcatheter aortic valve implantation using a balloon-expandable valve in Japan: experience from the Japanese pilot health technology assessment. Value Health Reg Issues. 2020;21:82–90. doi: 10.1016/j.vhri.2019.07.013.
- Ministry of Health Law. Outline of the revision of medical fees of 2022. https://www.mhlw.go.jp/content/12400000/001079187.pdf. (accessed March 21, 2023).
- Consumer Price Index/2020 Base Consumer Price Index/Annual Report: Price Statistics Office. Consumption Statistics Division, Statistical Research Department, Statistics Bureau, Japan; 2020 [30 March 2023]. Available from: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00200573&tstat=000001150147&cycle=7&tclass1=000001150150&stat_infid=000032177636&tclass2val=0.
- Hasegawa M, Komoto S, Shiroiwa T, et al. Formal implementation of cost-effectiveness evaluations in Japan: a unique health technology assessment system. Value Health. 2020;23(1):43–51. doi: 10.1016/j.jval.2019.10.005.
- Izumi C, Eishi K, Ashihara K, et al. JCS/JSCS/JATS/JSVS 2020 guidelines on the management of valvular heart disease. Circ J. 2020;84(11):2037–2119. doi: 10.1253/circj.CJ-20-0135.
- Madhavan MV, Kodali SK, Thourani VH, et al. Outcomes of SAPIEN 3 transcatheter aortic valve replacement compared with surgical valve replacement in intermediate-risk patients. J Am Coll Cardiol. 2023;82(2):109–123. doi: 10.1016/j.jacc.2023.04.049.
- Kodali S, Thourani VH, White J, et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk and intermediate-risk patients with aortic stenosis. Eur Heart J. 2016;37(28):2252–2262. doi: 10.1093/eurheartj/ehw112.
- Kodali S. SAPIEN 3 Transcatheter Aortic Valve Replacement Compared with Surgery inIntermediate risk Patients: a Propensity Matched Analysis of 5 year Outcomes2020 Transcatheter Valve Therapies (TVT) Summit; 18-20 June 2020; Virtual 2020.
- Makkar RR, Thourani VH, Mack MJ, et al. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med. 2020;382(9):799–809. doi: 10.1056/NEJMoa1910555.
- Gilard M, Eltchaninoff H, Iung B, et al. Cost-Effectiveness analysis of SAPIEN 3 transcatheter aortic valve implantation procedure compared with surgery in patients with severe aortic stenosis at low risk of surgical mortality in France. Value Health. 2022; 25(4):605–613. doi: 10.1016/j.jval.2021.10.003.
- Goodall G, Lamotte M, Ramos M, et al. Cost-effectiveness analysis of the SAPIEN 3 TAVI valve compared with surgery in intermediate-risk patients. J Med Econ. 2019;22(4):289–296. doi: 10.1080/13696998.2018.1559600.
- Tarride JE, Luong T, Goodall G, et al. A Canadian cost-effectiveness analysis of SAPIEN 3 transcatheter aortic valve implantation compared with surgery, in intermediate and high-risk severe aortic stenosis patients. Clinicoecon Outcomes Res. 2019;11:477–486. doi: 10.2147/CEOR.S208107.
- Baron SJ, Wang K, House JA, et al. Cost-effectiveness of transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at intermediate risk: results from the PARTNER 2 trial. Circulation. 2019;139(7):877–888. doi: 10.1161/CIRCULATIONAHA.118.035236.
- Galper BZ, Golden J, Rhee J, et al. Implementation of a post-transcatheter valve replacement (TAVR) fast-track care protocol with a focus on next-day discharge. J Am Coll Cardiol. 2018;71(11):A1224. doi: 10.1016/S0735-1097(18)31765-0.
- Heathcote L, Srivastava T, Sarmah A, et al. A systematic review and statistical analysis of factors influencing the cost-effectiveness of transcatheter aortic valve implantation for symptomatic severe aortic stenosis. Clinicoecon Outcomes Res. 2023;15:459–475. doi: 10.2147/CEOR.S392566.
- Herrmann HC, Thourani VH, Kodali SK, et al. One-year clinical outcomes with SAPIEN 3 transcatheter aortic valve replacement in high-risk and inoperable patients with severe aortic stenosis. Circulation. 2016;134(2):130–140. doi: 10.1161/CIRCULATIONAHA.116.022797.
- Ando T, Briasoulis A, Holmes AA, et al. Sapien 3 versus sapien XT prosthetic valves in transcatheter aortic valve implantation: a meta-analysis. Int J Cardiol. 2016;220:472–478. doi: 10.1016/j.ijcard.2016.06.159.
- Galper BZ, Chinnakondepalli KM, Wang K, et al. Economic outcomes of transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis and low surgical risk: results from the PARTNER 3 trial. Circulation. 2023;147(21):1594–1605. doi: 10.1161/CIRCULATIONAHA.122.062481.
- Kodera S, Kiyosue A, Ando J, et al. Cost effectiveness of transcatheter aortic valve implantation in patients with aortic stenosis in Japan. J Cardiol. 2018;71(3):223–229. doi: 10.1016/j.jjcc.2017.10.008.
- Shih YT, Liu L. Use of claims data for cost and cost-effectiveness research. Semin Radiat Oncol. 2019;29(4):348–353. doi: 10.1016/j.semradonc.2019.05.009.
- Daniel JB, Smriti S, Philip AM, et al. Long-Term durability of transcatheter aortic valve prostheses. J Am Coll Cardiol. 2019;73(5):537–545.
- Ando T, Kumamaru H, Kohsaka S, et al. Procedural volume and outcomes of transfemoral transcatheter aortic valve replacement: from a Japanese nationwide registry. Am J Cardiol. 2023;209:36–41. doi: 10.1016/j.amjcard.2023.09.094.
- Eerdekens R, Kats S, Grutters JP, et al. Cost-utility analysis of TAVI compared with surgery in patients with severe aortic stenosis at low risk of surgical mortality in The Netherlands. Cost Eff Resour Alloc. 2024;22(1):24. doi: 10.1186/s12962-024-00531-6.
- Baron SJ, Ryan MP, Moore KA, et al. Contemporary costs associated with transcatheter versus surgical aortic valve replacement in medicare beneficiaries. Circ Cardiovasc Interv. 2022;15(3):e011295.