Abstract
Objective
To compare the cost, healthcare utilization, and outcomes between skin and serum-specific IgE (sIgE) allergy testing.
Methods
This retrospective cohort study used IBM® MarketScan claims data, from which commercially insured individuals who initiated allergy testing between January 1 and December 31, 2018 with at least 12 months of enrollment data before and after index testing date were included. Cost of allergy testing per patient was estimated by testing pattern: skin only, sIgE only, or both. Multivariable linear regression was used to compare healthcare utilization and outcomes, including office visits, allergy and asthma-related prescriptions, and emergency department (ED) and urgent care (UC) visits between skin and sIgE testing at 1-year post testing (α = 0.05).
Results
The cohort included 168,862 patients, with a mean (SD) age of 30.8 (19.5) years; 100,666 (59.7%) were female. Over half of patients (56.4%, n = 95,179) had skin only testing, followed by 57,291 patients with sIgE only testing and 16,212 patients with both testing. The average cost of allergy testing per person in the first year was $430 (95% CI $426–433) in patients with skin only testing, $187 (95% CI $183–190) in patients with sIgE only testing, and $532 (95% CI $522–542) in patients with both testing. At 1-year follow-up post testing, there were slight increases in allergy and asthma-related prescriptions, and notable decreases in ED visits by 17.0–17.4% and in UC visits by 10.9–12.6% for all groups (all p < 0.01). Patients with sIgE-only testing had 3.2 fewer allergist/immunologist visits than patients with skin-only testing at 1-year follow-up (p < 0.001). Their healthcare utilization and outcomes were otherwise comparable.
Conclusions
Allergy testing, regardless of the testing method used, is associated with decreases in ED and UC visits at 1-year follow-up. sIgE allergy testing is associated with lower testing cost and fewer allergist/immunologist visits, compared to skin testing.
Introduction
Allergic diseases affect almost 1 in 3 adults and more than 1 in 4 children in the USCitation1,Citation2, imposing a considerable burden on individual and public healthCitation3,Citation4. Diagnosis and identification of sensitizing allergens is crucial for allergic disease management, including antigen immunotherapy, food and inhalant allergen avoidance and certain biologic treatmentsCitation5,Citation6. Additionally, determining atopy through testing helps differentiate allergic from non-allergic diseases with similar clinical presentations (e.g. non-allergic rhinitis and non-allergic food intolerance)Citation7–9.
There are currently two testing methods for diagnosing inhalant and food allergies: in vivo skin and in vitro serum allergen specific immunoglobin E (sIgE) testingCitation5,Citation6. Both tests may be employed to identify specific allergens with comparable sensitivity and clinical utilityCitation10,Citation11, with high concordance in detecting allergy sensitizationCitation12–15. US practice parameters for allergy diagnostic testing recommend either skin or sIgE testing be used in conjunction with clinical history when diagnosing allergic diseasesCitation10,Citation16.
Although the clinical properties of skin and sIgE tests are well documented, there is a lack of recent data on how the healthcare cost, utilization, and outcomes differ between the two tests. The only publicly available cost information is at the allergen level, based on the Medicare Physician and Clinical Laboratory Fee Schedule. In 2018, the per allergen cost was $5.40 for skin prick tests, $8.28 for intradermal skin tests, and $6.44 for sIgE testsCitation17,Citation18. A recent study of 100% Medicare fee-for-service beneficiaries in 2019 found that sIgE testing was associated with lower testing cost and fewer allergist visits per beneficiary, compared to skin prick testingCitation19. However, the study was cross-sectional and did not follow the same individuals over time or investigate in depth associated health care utilization patterns and outcomes, which are key to optimal diagnostic approaches. Based on the evidence available, we hypothesized that sIgE testing cost may be different than skin testing at the patient level while both allergy tests add clinical value to allergic disease identification and management. To test this hypothesis and fill in the knowledge gap, we utilized a large cohort of patients enrolled in U.S. commercial health insurance plans, with the primary aim to compare 1-year outcomes in healthcare utilization between patients receiving skin versus sIgE allergy testing, including physician office visits, urgent care (UC) visits, emergency department (ED) visits, and related medication prescriptions. The secondary aim was to compare cost at the patient level between skin versus sIgE testing.
Methods
Data source and study design
For this retrospective cohort study, we extracted 2017–2019 data from the IBM® MarketScan Commercial Claims and Encounters database and Medicare Supplemental and Coordination of Benefits database. These databases include de-identified administrative healthcare claims from a variety of health plans across the U.S., covering over 22 million individuals annually, for whom enrollment and demographic information as well as inpatient, outpatient, and pharmacy claims are available. More information about the MarketScan data series can be found in Hansen’s white paper published by IBM Watson HealthCitation20.
This study was exempt by the University of Southern California School of Medicine institutional review board under Category 4 of 45 CFR 46.101(2)(b) because it was based on existing deidentified secondary data. Information in the data was recorded in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. No consent to participate was required for this exempted research. All methods were performed in accordance with the relevant guidelines for secondary data analyses.
Cohort definition
The cohort consisted of patients who initiated at least one skin or sIgE allergy test in the identification period of January 1 to December 31, 2018. The first date of allergy testing claims during the identification period was identified as the index date for each patient. To establish a clear baseline for comparisons of associated health utilization patterns and testing cost, patients were required to be continuously enrolled (gaps in enrollment at any point < = 45 days) without any allergy testing claims for at least 12 months before the index date. To ensure adequate follow-up, patients were followed up after index date for at least 12 months.
Measures
The primary outcome was cost of allergy testing and associated healthcare resource utilization. Eligible allergy tests were identified using Current Procedural Terminology (CPT) codes, specifically 86003 or 86008 for sIgE tests, and 95004 or 95024 for skin tests (inclusive of skin prick test and intradermal skin tests). Patch testing to identify type IV hypersensitivity reactions, such as allergic contact dermatitis, was not included where sIgE testing is not applicable. Direct costs of the allergy testing procedures, including plan paid amount and patient out-of-pocket cost such as co-payment, co-insurance and deductibles, were extracted from claim line items associated with the aforementioned CPT codes, and aggregated at the patient level. Baseline and first-year healthcare utilization post index date, including number of relevant office visits and number of allergy-related prescriptions, were also measured. Office visits included those with primary care physicians, allergists/immunologists, and pulmonologists. Allergy-related prescriptions included corticosteroids, asthma relievers, asthma controller, and biologics indicated for the treatment of severe asthma. Secondary outcomes included number of UC and ED visits in the first-year post index date.
The key independent variable was the utilization patterns of allergy testing, namely, skin only testing, sIgE only testing, or both, in the identification period. Baseline characteristics included age group (0–4, 5–11, 12–18, 19–64, and 65+ years of age respectively), sex, insurance plan type, place of service, and specialty of provider who ordered the index allergy test. Race and ethnicity information were not available in the databases and therefore not included in the analyses.
Statistical analysis
Baseline characteristics were summarized by allergy testing utilization type and sequence at the patient level: skin only testing, sIgE only testing, skin followed by sIgE testing, sIgE followed by skin testing, or both skin and sIgE concurrently. Among other outcome measures, allergy testing cost per patient, solely on the testing procedures and not including physician fees, was compared by allergy testing type: skin only testing, sIgE only testing, and both. Independent-samples t-test was used to compare each pair of testing types on continuous outcome measures. Two-sample Z Proportion test was used to compare outcome measures in proportions. Paired-samples t-test was used to compare outcomes at baseline vs 1-year within each patient subgroup by testing type. The significance level was set at 0.05 for all tests. For all patients, we conducted multivariable linear regression to estimate the association of allergy testing type and each of the one-year healthcare utilization outcome measures, adjusting for age, sex, and all baseline healthcare utilization outcome measures in the year prior to index testing. This model specification was theory driven, based on the following reasoning: (1) a parsimonious model is typically preferred and thus we included only key characteristics such as age and sex (race/ethnicity was not available in the data); (2) clinical variables such as place of service and specialty of provider were strongly correlated with type of allergy testing, the key predictor, and were therefore not included as potential confounders; (3) baseline key healthcare utilization and outcome measures were included to adjust for prior differences between patients who utilized skin tests vs. sIgE testing that were often not observable and were related to their general utilization and outcome patterns such as clinical history and disease severity. All analyses were conducted with SAS statistical software version 9.4 (Cary, NC).
Results
Sample
Our cohort consisted of 168,862 patients, with a mean (SD) age of 30.8 (19.5) years; 100,665 (59.7%) were female. Across the whole cohort, 8.1% of patients were 0 - 4 years of age (n = 13,659), 15.1% were between 5 - 11 (n = 25,528), 12.6% were 12 - 18 (n = 21,191), 63.1% were 19 - 64 (n = 106,350), and 1.2% were 65 or older (n = 1,954) (). The primary health insurance plan was HMO for 15.7% of patients and PPO for 44.2% of patients; the rest had other (37.6%) and unidentified (2.5%) plans.
Table 1. Summary statistics by allergy testing type.
In terms of allergy testing patterns, 56.4% of patients used skin only testing (n = 95,179), the most common method, followed by 34.0% with sIgE only testing (n = 57,291), and 9.6% with both skin and sIgE testing (n = 16,212). Among those with both skin and sIgE testing, 5,345 had skin testing before sIgE testing, 5,946 had sIgE testing before skin testing, and 4,921 had both skin and sIgE testing concurrently.
Healthcare utilization and outcomes
At baseline (0–12 months before index testing), utilization of relevant prescriptions was similar between patients with skin only testing and those with sIgE only testing. Specifically, the mean ± SD were 0.56 ± 1.11 vs. 0.51 ± 1.2 in number of oral corticosteroid prescriptions (p = 0.007), 0.44 ± 1.19 vs. 0.44 ± 1.31 in number of asthma reliever prescriptions (p = 0.644), 0.33 ± 1.24 vs. 0.33 ± 1.33 in number of asthma controller prescriptions (p = 0.502), and 0.01 ± 0.27 vs. 0.01 ± 0.34 in number of biologics prescriptions (p = 0.005). In terms of clinical encounters, patients with skin only testing had slightly more PCP visits (2.5 vs. 2.37), allergist/immunologist visits (0.71 vs. 0.31), and UC visits (0.25 vs. 0.22), and slightly fewer ED visits (0.33 vs. 0.38) than those with sIgE only testing, all p < 0.001 ().
Table 2. Unadjusted health utilization and outcomes at baseline and 1-year post index testing, by allergy testing patterns.
Within each of the three patient subgroups by testing pattern, including skin only, sIgE only, and both skin and sIgE, the number of oral corticosteroid prescriptions, UC visits, and ED visits decreased following index allergy testing. Specifically, the changes from baseline to 1-year follow up were: oral corticosteroid prescriptions decreased by 18.5% (from 0.56 to 0.46) in patients with skin testing and by 9.8% (from 0.51 to 0.46) in patients with sIgE testing; UC visits decreased by 12.6% (from 0.25 to 0.21) in patients with skin testing and by 10.9% (from 0.22 to 0.20) in patients with sIgE testing; ED visits decreased by 17.0% (from 0.33 to 0.27) in patients with skin testing and by 17.4% (from 0.38 to 0.31) in patients with sIgE testing, all p < 0.01. In contrast, the number of asthma controller and biologic prescriptions increased for all 3 subgroups after allergy testing, all p < 0.01 ().
Unadjusted 1- year results of between-group comparisons showed higher proportions of patients with skin only testing were prescribed oral corticosteroids, asthma relievers, and asthma controllers, compared to patients with sIgE only testing. The mean (95% CI) percentages were 27.0 (26.3 − 27.7) vs 25.2 (24.4 − 25.9), 22.1 (21.3 − 22.9) vs 19.3 (18.5 − 20.1), 15.1 (14.2 − 15.9) vs. 12.6 (11.7 − 13.4), respectively, all p < 0.001) (). However, patients with sIgE only testing were slightly more likely to have been prescribed biologics than patients with skin only testing (0.9% (0 − 1.9) vs. 0.6% (0 − 1.6), p < 0.001).
Figure 1. Health care utilization and outcomes 0-12 months post index allergy testing, per patient by testing pattern. (a) Prescriptions, (b) Visits.
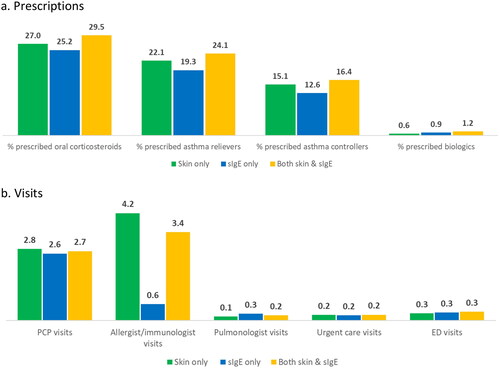
In terms of healthcare utilization within 12 months of the index date, unadjusted outcomes were comparable across all three groups of patients by testing pattern, in PCP visits, pulmonologist visits, UC visits, and ED visits (). The most notable difference was in allergist/immunologist visits, where the mean (95% CI) of visits was 4.2 (4.1 − 4.2) in patients with skin only testing, 0.6 (0.6 − 0.7) in patients with sIgE only testing, and 3.4 (3.3 − 3.6) in patients with both skin and sIgE testing (all p < 0.001).
The multivariable linear regression results on the healthcare utilization and outcomes were consistent with the unadjusted analyses (). At 1-year post index date, the differences between patients with skin only testing and those with sIgE only testing were marginal in all outcomes, except for allergist/immunologist visits, where patients with sIgE only testing had 3.2 fewer visits than patients with skin only testing (p < 0.001) ().
Table 3. Multivariable linear regression results on the associations between allergy testing patterns and subsequent health utilization and outcomes.
Testing cost
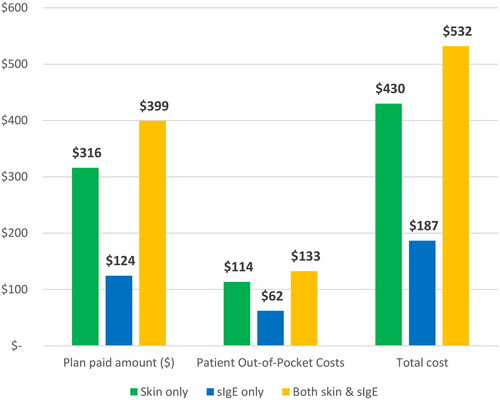
The mean (95% CI) cost of allergy testing per person, including and within 12 months of the index date was $430 (426 − 433) in patients with skin only testing, $187 (138 - 190) in patients with sIgE only testing, and $532 (522 – 542) in patients with both skin and sIgE testing (). Health plan reimbursement accounted for the majority of the cost, and was $316 (314 - 318) in patients with skin only testing, $124 (122 -127) in patients with sIgE only testing, and $399 (393 − 406) in patients with both skin and sIgE testing (all p < 0.001).
Discussion
To the best of the authors’ knowledge, there have been no recent studies comparing skin versus sIgE tests for the diagnosis and management of allergic diseases in terms of healthcare utilization and clinical outcomes, despite various practice parameters recommending that either forms of testing can be used. Our retrospective cohort study is the first to demonstrate the following:
First, allergy testing is associated with lower utilization of urgent and emergency care services in the subsequent year despite testing methods, suggesting both skin and sIgE allergy testing methods are beneficial in allergy disease management (). Allergy-related clinical metrics also improved at 1 year after allergy testing, as indicated by increases in asthma controller and biologic prescriptions, which are effective in reducing allergic inflammationCitation9,Citation21, and therefore may at least in part explain the reduced use of UC and ED services. In summary, sIgE testing is of comparable efficacy to skin testing in guiding management of allergic disease.
Second, commercially insured patients utilizing different methods of allergy testing are similar in demographics at baseline (). Further, we find that their relevant healthcare utilization and clinical outcomes were also mostly comparable at both baseline and at 1-year follow-up. There were slightly more ED visits (0.38 vs. 0.33) and slightly fewer UC visits (0.22 vs. 0.25) in patients with sIgE testing compared to patients with skin testing at baseline. These clinically insignificant differences were statistically significant due to the large sample sizes, and may be partially explained by variations in the availability of care options by geographical location. Notably, individuals living in rural areas have less access to UC than ED servicesCitation22,Citation23. They may also be more likely to utilize sIgE testing due to geographical barriers in accessing allergistsCitation24,Citation25. This is consistent with our data, showing significantly fewer allergist/immunologist visits among sIgE only patients than patients with skin only testing at baseline (0.31 vs. 0.71).
Third, sIgE only testing was associated with significantly lower costs than skin testing at the individual person level, in total reimbursements per patient ($187 vs. 430, ), including health plan and patient out-of-pocket payments. As commercial insurance payment often parallels the Medicare fee schedule, the cost per allergen in our study sample may have been comparable between skin testing (inclusive of skin prick and intradermal) and sIgE testing, but a higher number of allergens may have been tested in patients with skin only testing compared to those with sIgE only testing, resulting in higher overall testing costs for the former. This finding is consistent with a cross-sectional study of 100% Medicare fee-for-service beneficiaries in 2019 that, per patient, sIgE testing incurs lower costs than skin testing and is associated with fewer allergens tested, compared to skin testingCitation19. Our study demonstrates that the significantly lower costs of sIgE testing applies to various commercially insured patient populations in addition to that of Medicare.
Besides the cost differences at the patient level, skin and sIgE testing also differ in delivery modality. Specifically, skin tests are typically performed and reported by specialists such as allergists in a clinical setting. In contrast, sIgE tests can be prescribed by a wide range of providers and are performed, processed, and reported by clinical laboratoriesCitation6,Citation10,Citation11. The differences in prescribing patterns by provider specialty are confirmed by our findings in . Cox et al. in the ACAAI/AAAAI Specific IgE Task Force report explained that allergists usually perform skin prick tests due to rapidly available results, reproducibility, and minimal invasivenessCitation16. Consequently, our data show that at 1-year post index testing, the number of primary care visits was essentially the same by testing type; however, patients who had skin testing only visited an allergist/immunologist 3.2 more times than patients who had skin testing only (). These additional allergist visits by patients with skin only testing could be follow up visits for further allergy consultation and management. Additional cost and value may be associated with these visits but it is beyond the scope of this paper to make such evaluations. This also suggests the actual cost savings associated with sIgE testing may be far greater due to a lack of professional fees, which may translate to future premium decreases.
Aside from healthcare expenditure savings, wider use of sIgE testing will help address the increasing burden of atopic disease in the U.S. Global shifts to urban lifestyles, microbiome and climate changes have resulted in rising incidence of atopic disease and prolonged exposure of vulnerable individuals to allergensCitation26–29. Additionally, there is a significant shortage of allergy specialists in the U.S. especially in rural and under-served communities, reducing access and delaying allergy skin testingCitation24,Citation25,Citation30. The unmet needs of more vulnerable patients requiring allergy testing due to specialist shortages and increasing disease prevalence can in part be ameliorated through increased access and use of sIgE testing in primary care settings in conjunction with strategies to increase access to allergy specialists (e.g. use of sIgE tests in telemedicine consults with specialists).
Our study had a few limitations. First, the study cohort included patients with a primary diagnosis of drug and stinging insect allergy in whom skin testing is the preferred modality in the analysis. However, this subgroup only constituted 0.6% of our study cohort and did not substantively change the overall results. Second, although skin and sIgE tests are similarly accurate, they are not identical. Skin test panels often include several types of similar allergens (e.g. grasses) that cross react and are represented by a single allergen on sIgE tests. It is unclear whether the greater variety of allergens included in skin tests could provide additional clinical benefits. Third, we could only estimate the cost of the allergy tests themselves and not the expenditures associated with physician office visits. This was because physician visits directly associated with sIgE test prescriptions could not be ascertained due to limitations of claims data. Nevertheless, our findings on the use of primary care and relevant specialist visits in the follow-up period provide useful context regarding the overall cost of allergy testing by type. Fourth, we did not have information on what specific allergic symptoms or diseases patients were tested for. Certain outcome measures we selected were more relevant to allergic asthma because they could be estimated directly. We acknowledge that allergy testing is also performed for other IgE-mediated diseases, including urticaria, atopic dermatitis, and food allergies. Lastly, the quality of both skin and sIgE testing varies, and the varying quality cannot be captured in the claims data. For skin tests specifically, the quality of extracts varies by manufacturer, especially when food and fungal extracts are concernedCitation31–36. In addition, the interpretation of skin test results could also vary by placement of tests, device used, technician performance of tests, and interference of other medicationsCitation16. For sIgE tests, the quality varies by manufacturer. However, the ImmunoCAP sIgE blood test, the gold standard in allergy blood testing, has an over 80% market share in the U.SCitation37. All major commercial laboratories such as Quest, Labcorp, and Kaiser utilize ImmunoCAP testing technology. The large presence of ImmunoCAP tests greatly reduces variability of sIgE test results in general in the US.
Conclusions
In conclusion, our study found patients had fewer UC and ED visits, at 1-year after initial allergy testing, regardless of the allergy testing methods utilized. Compared to patients who only received skin testing, patients who received sIgE testing exclusively for allergic sensitization had lower costs and fewer allergist/immunologist visits, but mostly comparable healthcare utilization and outcomes at 1-year follow up.
Transparency
Declaration of financial/other relationships
The authors declare no financial/other interests. The funder had no role in the design and conduct of the study, or the collection, management, analysis, and interpretation of the data.
Author contributions
YZL was responsible for the study design, analysis of the data, interpretation of results and writing of the manuscript, and provided critical revisions to the manuscript. KYK was responsible for the study concept, study design, interpretation of results and writing of the manuscript, and provided critical revisions to the manuscript. Both authors reviewed the manuscript, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work. This manuscript has not been published elsewhere and is not currently under consideration by another journal.
Acknowledgements
The authors thank Thomas Goss and Manasi Datar of Veranex for their assistance in data acquisition and analysis, and the 3 anonymous reviewers for their valuable feedback.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they have performed consulting, served on advisory boards, or received travel reimbursement from Amphastar, AstraZeneca, Chiesi, Connect Biopharma, GlaxoSmithKline, Mylan, Novartis, Sunovion and Theravance. They have also conducted multicenter clinical research trials for some 40 pharmaceutical companies.
The other peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Additional information
Funding
References
- Zablotsky B, Black LI, Akinbami LJ. Diagnosed Allergic Conditions in Children Aged 0–17 Years: united States, 2021. NCHS data brief; no. 459. In: national Center for Health S, editor. Hyattsville, MD: doi:10.15620/cdc:123250.; 2023.
- Ng AE, Boersma P. Diagnosed allergic conditions in adults: United States, 2021. NCHS Data Brief, no 460. Hyattsville, MD: national Center for Health Statistics 2023.
- Gupta R, Sheikh A, Strachan DP, et al. Burden of allergic disease in the UK: secondary analyses of national databases. Clin Exp Allergy. 2004;34(4):520–526. doi:10.1111/j.1365-2222.2004.1935.x.
- Pawankar R. Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J. 2014;7(1):12. doi:10.1186/1939-4551-7-12.
- Adkinson NF, Jr., Hamilton RG. Clinical history-Driven diagnosis of allergic diseases: utilizing in vitro IgE testing. J Allergy Clin Immunol Pract. 2015;3(6):871–876. doi:10.1016/j.jaip.2015.08.002.
- Hamilton RG. 96 - Assessment of human allergic diseases. In: Rich RR, Fleisher TA, Schroeder HW, et al. editors. Clinical immunology. 6th edn. Elsevier; 2023. p. 1225–1233.
- Hellings PW, Klimek L, Cingi C, et al. Non-allergic rhinitis: position paper of the european academy of allergy and clinical immunology. Allergy. 2017;72(11):1657–1665. doi:10.1111/all.13200.
- Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014;134(5):1016–1025.e43. doi:10.1016/j.jaci.2014.05.013.
- Burks AW, Holgate ST, O'Hehir RE, et al. Middleton’s allergy: principles and practice. Elsevier Health Sciences; 2019.
- Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(3 Suppl 3):S1–S148. doi:10.1016/s1081-1206(10)60305-5.
- Hamilton RG, Hemmer W, Nopp A, et al. Advances in IgE testing for diagnosis of allergic disease. J Allergy Clin Immunol Pract. 2020;8(8):2495–2504. doi:10.1016/j.jaip.2020.07.021.
- Bignardi D, Comite P, Mori I, et al. Allergen-specific IgE: comparison between skin prick test and serum assay in real life. Allergol Select. 2019;3(1):9–14. doi:10.5414/ALX01891E.
- Knight V, Wolf ML, Trikha A, et al. A comparison of specific IgE and skin prick test results to common environmental allergens using the HYTEC™ 288. J Immunol Methods. 2018;462:9–12. doi:10.1016/j.jim.2018.07.005.
- Ciprandi G, De Amici M, Giunta V, et al. Comparison of serum specific IgE and skin prick test in polysensitized patients. Int J Immunopathol Pharmacol. 2010;23(4):1293–1295. doi:10.1177/039463201002300438.
- Klemans RJ, van Os-Medendorp H, Blankestijn M, et al. Diagnostic accuracy of specific IgE to components in diagnosing peanut allergy: a systematic review. Clin Exp Allergy. 2015;45(4):720–730. doi:10.1111/cea.12412.
- Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American college of allergy, asthma and immunology/American academy of allergy, asthma and immunology specific IgE test task force. Annals of Allergy, Asthma & Immunology. 2008;101(6):580–592. doi:10.1016/S1081-1206(10)60220-7.
- Centers for Medicare & Medicaid Services. Medicare Physician Fee Schedule 2018. https://www.cms.gov/medicare/physician-fee-schedule/search?Y=7&T=4&HT=1&CT=0&H1=95004&H2=95024&M=5
- Centers for Medicare & Medicaid Services. Medicare Clinical Laboratory Fee Schedule 2018. https://www.cms.gov/medicare/payment/fee-schedules/clinical-laboratory-fee-schedule-clfs
- Kwong KY, Lu YZ. Cost of serum versus skin allergy testing among medicare fee-for-service beneficiaries in the United States. J Health Econ Outcomes Res. 2023;10(2):14–21. doi:10.36469/001c.77482.
- Hansen L. IBM MarketScan research databases for life sciences researchers. IBM Watson Health; 2018.
- Pepper AN, Hanania NA, Humbert M, et al. How to assess effectiveness of biologics for asthma and what steps to take when there is not benefit. J Allergy Clin Immunol Pract. 2021;9(3):1081–1088. doi:10.1016/j.jaip.2020.10.048.
- Ayers A. Rural and tertiary markets: the next urgent care frontier. J Urgent Care Med. 2019.
- Muelleman RL, Sullivan AF, Espinola JA, et al. Distribution of emergency departments according to annual visit volume and urban-rural status: implications for access and staffing. Acad Emerg Med. 2010;17(12):1390–1397. doi:10.1111/j.1553-2712.2010.00924.x.
- Mann RM. Response to “allergist report: america faces an allergy/asthma crisis”. Ann Aller Asthma Immunol. 2007;99(5):470. doi:10.1016/S1081-1206(10)60576-5.
- Sun D, Heimall J. Geographical distribution of allergy/immunology providers in the United States and association with median income. J Allergy Clin Immunol Pract. 2020;8(8):2802–2804.e1. doi:10.1016/j.jaip.2020.04.018.
- von Mutius E. The rising trends in asthma and allergic disease. Clin Exp Allergy. 1998;28(Suppl 5):45–49. discussion 50-1. doi:10.1046/j.1365-2222.1998.028s5045.x.
- Asher MI, García-Marcos L, Pearce NE, et al. Trends in worldwide asthma prevalence. Eur Respir J. 2020;56(6):2002094. doi:10.1183/13993003.02094-2020.
- Barnes CS. Impact of climate change on pollen and respiratory disease. Curr Allergy Asthma Rep. 2018;18(11):59. doi:10.1007/s11882-018-0813-7.
- Beggs PJ. Climate change, aeroallergens, and the aeroexposome. Environ Res Lett. 2021;16(3):035006. doi:10.1088/1748-9326/abda6f.
- Allergist Report: america Faces an Allergy/Asthma Crisis. American College of Allergy, Asthma & Immunology. Arlington Heights, IL. 2007.
- Heinzerling L, Mari A, Bergmann K-C, et al. The skin prick test – european standards. Clin Transl Allergy. 2013;3(1):3. doi:10.1186/2045-7022-3-3.
- Goodman RE, Chapman MD, Slater JE. The allergen: sources, extracts, and molecules for diagnosis of allergic disease. J Allergy Clin Immunol Pract. 2020;8(8):2506–2514. doi:10.1016/j.jaip.2020.06.043.
- Slater JE, Menzies SL, Bridgewater J, et al. The US food and drug administration review of the safety and effectiveness of nonstandardized allergen extracts. J Allergy Clin Immunol. 2012;129(4):1014–1019. doi:10.1016/j.jaci.2012.01.066.
- Codina R, Esch RE, Lockey RF. The clinical relevance of pollen versus fungal spores in allergic diseases. The. J Allergy Clin Immunol Pract. 2021;9(10):3615–3620. doi:10.1016/j.jaip.2021.06.004.
- Ruethers T, Taki AC, Nugraha R, et al. Variability of allergens in commercial fish extracts for skin prick testing. Allergy. 2019;74(7):1352–1363. doi:10.1111/all.13748.
- Hauck PR, Williamson S. The manufacture of allergenic extracts in North america. Clin Rev Allergy Immunol. 2001;21(2-3):93–110. doi:10.1385/CRIAI:21:2-3:93.
- Going beyond traditional allergy and autoimmune testing [Internet]. 2012. www.thermoscientific.com/content/dam/tfs/SDG/IDD/PiRL/PiRL%20Sell%20Sheet%20070512.pdf