Abstract
Objective: This study aimed to compare the efficacy and safety of ossein-hydroxyapatite complex (OHC) versus calcium carbonate (CC) for preventing bone loss during perimenopause in current clinical practice.
Methods: The prospective, comparative, non-randomized, open-label study included 851 perimenopausal women with basal bone mineral density (BMD) T-score ≥−2 standard deviations (SDs). Participants received either OHC (712 mg calcium/day) or CC (1000 mg calcium/day) over 3 years. BMD was evaluated by dual-energy X-ray absorptiometry at the lumbar spine (L2–L4) at baseline and after 18 and 36 months of follow-up. Adverse drug reactions (ADRs) were also recorded.
Results: In women receiving OHC, BMD at the L2–L4 site remained stable over the 3-year follow-up period (mean [SD] change 0.00 [0.11] g/cm2). BMD in the CC arm decreased −3.1% (mean [SD] − 0.03 [0.11] g/cm2). Between-group differences were statistically significant (p < 0.001) and favored OHC. ADRs were more frequent in the CC group (7.7% vs. 2.7% in the OHC group; p = 0.001), affecting primarily the gastrointestinal system.
Conclusion: OHC showed greater efficacy and tolerability than CC for bone loss prevention in perimenopausal women in real-world practice. As the daily dose of calcium was higher in the CC group, the differences might be linked to the ossein compound in OHC.
摘要
目的:本研究旨在比较骨胶原-羟基磷灰石复合物(OHC)与碳酸钙(CC)在当前临床实践中预防围绝经期骨质流失的有效性和安全性。
方法:此前瞻性, 比较性, 非随机, 开放性研究包括851名围绝经期妇女, 其基础骨密度(BMD)T评分≥-2标准差(SD)。在3年的时间里, 参与者接受OHC(712毫克钙/天)或接受CC(1000毫克钙/天)治疗。在基线和随访18和36个月后, 采用双能x线骨密度仪测定腰椎(L2-L4)的骨密度, 同时记录不良反应(ADRs)。
结果:在接受OHC的女性中, L2-L4部位的BMD在3年的随访期内保持稳定(平均[SD]变化0.00[0.11]g/cm2)。CC组BMD下降-3.1%(平均[SD] -0.03[0.11] g/cm2)。组间差异有统计学意义(p<0.001;OHC效果更佳)。不良反应主要影响胃肠道系统, 在CC组更常见(7.7% VS. 2.7% OHC组);p= 0.001)。
结论:在现实生活中, OHC在围绝经期妇女预防骨质流失方面比CC表现出更大的有效性和耐受性。由于CC组的钙日剂量较高, 其差异可能与OHC中的骨胶原化合物有关。
Introduction
The World Health Organization describes osteoporosis as a ‘progressive systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture’Citation1. The increased rate of fractures associated with the disease is one of the most common causes of disability and a major contributor to medical care costs in many regions of the worldCitation2. A recent systematic review showed that insufficient calcium intake is a worldwide health problem with potentially serious consequences, particularly in women and especially given the aging of the populationCitation3.
Research on osteoporosis in women has focused primarily on the postmenopausal and elderly period. Nevertheless, an accelerated rate of bone loss has also been reported during the menopausal transitionCitation4,Citation5, when estrogen secretion is markedly reduced particularly in the year before the final menstrual period and the first 2 years thereafterCitation6.
Calcium, at a recommended daily intake of 700–1200 mgCitation7,Citation8, is an important adjunctive therapy for the treatment and prevention of osteoporosis when dietary intake is insufficientCitation7–9. Meta-analysis has shown it to be more effective than placebo in reducing bone loss by the second year of treatmentCitation10, and that it has a positive effect on bone mineral density (BMD) and a tendency to reduce fracture incidence.
Calcium carbonate (CC) is one widely used calcium supplement when the recommended dietary calcium intake is insufficient. Another supplement is ossein-hydroxyapatite complex (OHC), which has also been shown to be effective in maintaining BMD and to have a more intense osteogenic effect than a calcium supplement alone after oral administrationCitation11–18. OHC consists of ossein, the protein that forms the organic matrix of bone, and hydroxyapatite (Ca5[PO4]3OH), the most relevant bone salt of vertebrate bone.
Little is known about the comparative effectiveness of calcium supplements in the perimenopausal period. However, based on results from clinical trialsCitation14–18, our hypothesis was that OHC might also be more effective than CC at preventing bone loss in perimenopause. The aim of the present study was therefore to compare the efficacy, safety, and tolerability of OHC versus CC in preventing bone loss in perimenopausal women over a 3-year treatment period in conditions of usual clinical practice.
Methods
Study design and population
The PRevention of the Osteoporosis at the Perimenopausal period (PROP) trial (ISRCTN83573042) was a Strengthening The Reporting of OBservational Studies in Epidemiology (STROBE)-compliant observational, prospective, multicenter, open-label study performed between 2005 and 2014 in accordance with the Declaration of Helsinki (2004) and local legislation on data protection. Participants were followed for up to 3 years and made a total of seven study visits (baseline and 6, 12, 18, 24, 30, and 36 months).
Participants were recruited consecutively in outpatient gynecology clinics around Spain. As safety was one of the main objectives of the study, and due to the relatively limited amount of tolerability data available for OHC, it was planned to include participants at a ratio of 3:1 for OHC and CC, respectively. Women were eligible to participate if they met the following criteria: 40–50 years of age; perimenopausal at study commencement; and lumbar or hip BMD T-score ≥−2 standard deviations (SDs) (normal BMD or mild osteopenia) measured using dual-energy X-ray absorptiometry (DXA) likely, in the opinion of the attending clinician, to benefit from calcium supplementation. A participant was considered perimenopausal if she reported menstrual irregularity lasting less than 12 consecutive months not necessarily associated with menopausal symptoms such as hot flushes, vaginal dryness, or night sweats.
Patients were excluded if they had osteoporosis (BMD T-score ≤−2.5 SDs diagnosed using DXA) or severe osteopenia (BMD T-score <−2 SDs), if they were being treated with drugs with a known effect on bone metabolism (glucocorticoids, steroids, thyroid hormones, heparin [long-term treatment], anticonvulsants, contraceptives, hormone replacement therapy, lithium, cancer chemotherapy, selective estrogen receptor modulators, calcium supplements, vitamin D, immunosuppressive therapy, or bisphosphonates), or if they had a diagnosis of hypercalcemia, hypercalciuria, or neoplasia during the previous 5 years or osteomalacia, Paget bone disease, or diseases affecting bone metabolism. Pregnant women, as well as those with reporting hypersensitivity to any of the study drugs, or participants with gastrointestinal disturbances that could interfere with drug absorption, were also excluded.
Following a prescreening visit, all selected patients underwent bone densitometry using DXA. Patients meeting the selection criteria were included in the study after providing informed consent.
Given that this was an observational study performed under conditions of current clinical practice, no formal sample size calculation was carried out. However, upon completion of the study, statistical power was calculated based on the final number included and results from previous studiesCitation17, and was found to be sufficient to detect a difference between the two treatment groups of at least 2% in lumbar BMD after 3 years with a power of 85% and a significance level of 5%, using the Student t-test for independent data.
Treatment
Based on the criteria of the participating clinicians, participants received either OHC at a dose of two 830-mg tablets every 12 h (712 mg of elemental calcium per day) or CC at a dose of a single 1250-mg tablet every 12 h (equivalent to a total daily dose of 1000 mg of elemental calcium).
Of note, a single 830-mg OHC tablet (Osteopor®/Ossopan®/Osteogenon®/Totalos Plus®; Pierre Fabre Médicament, Castres, France) contains calcium (178 mg), phosphorus (82 mg), and proteins associated with bone metabolism (osteocalcin, 5.8 µg; type I collagen, 216 mg; insulin growth factor type I, 168 ng; insulin growth factor type II, 84 ng; transforming growth factor-β, 21 ng).
Assessments
Change in BMD was measured by DXA at the lumbar spine (L2–L4) at baseline and after 18 and 36 months of follow-up. When possible, BMD assessments for the femoral neck, trochanter, Ward’s triangle, and total hip were also sought. All bone densitometries (baseline and follow-up) were performed at the nearest reference center for each patient.
Adverse drug reactions (ADRs) associated with the treatments were recorded, as well as the number of patients with dose reduction due to toxicity and the number of patients withdrawing due to treatment intolerance. Participating clinicians directly questioned patients on the presence of ADRs and recorded their severity, duration, potential relation with the study drug, action taken, and outcome.
The presence of fractures, the body mass index, and risk factors for bone loss were recorded at the baseline visit. Daily dietary calcium intake was estimated using a questionnaire to record weekly average consumption of the most frequent food products.
At follow-up clinic visits, in addition to DXA assessments, changes in concomitant medication, withdrawal from treatment, and treatment compliance were recorded. Treatment compliance was evaluated at each 6-month follow-up visit by clinician interview. Patients were asked whether they took the medication daily and whether they took the medication at the prescribed dose. Possible responses for both questions were: 1 = ‘Never’, 2 = ‘Almost never’, 3 = ‘Almost always’, and 4 = ‘Always’. Patients were categorized as compliant (>70% of doses taken) if they answered ‘Almost always’ or ‘Always’ to both questions.
Statistical analysis
Descriptive statistics used absolute values and proportions, means, or medians as appropriate, together with measures of central tendency (SDs and interquartile ranges). Baseline characteristics for the two treatment groups were compared using Student’s t-test or the Mann–Whitney test and the chi-square or Fisher’s exact test as appropriate.
The safety analysis was conducted on the safety population, defined as all participants who took at least one dose of the study medication. The cumulative incidence of each type of ADR by cohort was estimated by dividing the number of patients with at least one recorded ADR by the total number of exposed participants over the study. Cumulative incidences were compared between groups using the chi-square or Fisher’s exact test.
The initial efficacy analysis was performed in the full analysis set; that is, patients who fulfilled all selection criteria, had taken the prescribed treatment with any degree of compliance, and had at least one efficacy assessment. A second efficacy analysis was performed using data from patients with an average compliance of 70% or more. The analysis was then repeated in both the overall and compliant-only populations after excluding patients who began taking any of the concomitant treatments described in the exclusion criteria (e.g. hormone replacement therapy, contraceptives, or isoflavones) while in the study. Treatment compliance was also analyzed and compared across study arms.
Change in bone mass was assessed based on mean DXA scores and expressed in grams per square centimeter and as T-scores. Paired t-tests or Wilcoxon tests were used as appropriate to assess the statistical significance of any changes.
Analyses were performed using SAS v9.4 (SAS Institute Inc, Cary, NC, USA), and all tests were two-sided with a significance level of 0.05.
Results
A total of 1032 women were considered eligible for inclusion in the study (). Of these, 181 were excluded from analysis because they failed to present at the first follow-up visit (n = 127 [16.8%] in the OHC group and n = 54 [19.6%] in the CC group). Data from 851 women were therefore available for the safety analysis (n = 629 in the OHC group and n = 222 in the CC group). Of those who began the study, 722 (69.9%) patients completed 1 year of follow-up, 534 (51.7%) patients completed 2 years, and 437 (42.3%) patients completed 3 years. Reported reasons for discontinuation in the OHC and CC groups, respectively, were: loss to follow-up, 83% and 61%; concomitant disease, 10% and 6%; and adverse effects, 7% and 33%.
For the efficacy analysis, 845 patients (99.3%) were included in the full analysis set (624 in the OHC group and 221 in the CC group); 487 (57.6%) patients were eligible for treatment-compliant analysis (n = 355 of the 624 patients [56.9%] in the OHC group and 132 of the 221 patients [59.7%] in the CC group). A total 57.6% (n = 487) of patients reported a level of compliance, representing over 70% of theoretical doses, with no statistically significant differences between treatment groups.
There were no significant differences between the OHC and CC groups for any of the baseline variables studied ().
Table 1. Baseline characteristics of the two study groups.
Change in bone mineral density
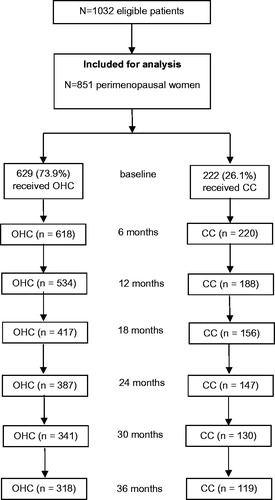
presents the baseline BMD at the lumbar spine (L2–L4) and the change after 36 months of treatment in the full analysis set population. The mean (SD) T-score in the CC group decreased by −0.23 (0.76) over the study period, compared to 0.01 (0.82) in the OHC group (difference in change scores significant at p < 0.001). Over the same period, bone density decreased by a mean (SD) of −0.03 (0.11) g/cm2 in the CC group but remained stable in the OHC group (mean [SD] change 0.00 [0.11] g/cm2), with the difference significant at p < 0.001 (). After 3 years of treatment, bone loss in the CC arm was 3.1% as shown in (p < 0.001 for the difference between study groups).
Figure 2. Lumbar T-score change (mean) from baseline in the study arms (L2–L4). CC, calcium carbonate; OHC, ossein-hydroxyapatite complex.
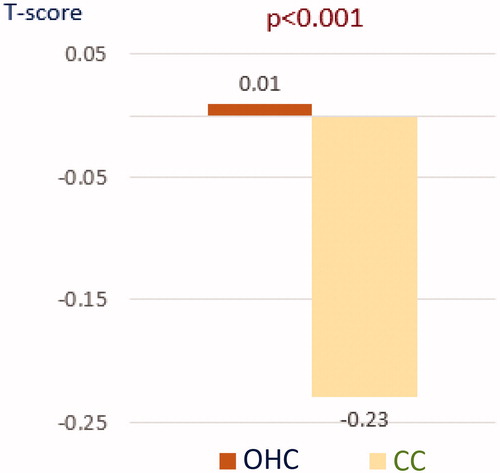
Figure 3. Mean percentage change from baseline in the study arms (L2–L4). CC, calcium carbonate; OHC, ossein-hydroxyapatite complex.
Table 2. Evolution of bone mineral density (T-scores and grams per square centimeter) at the lumbar spine (n = 807).
Changes in T-score and percentage changes at the lumbar site are illustrated graphically for the two groups in and .
Calcium consumption
The mean (SD) daily calcium consumption for the full sample was estimated at 993 (± 495) mg with no significant differences between the arms (). There was considerable variability in intake among participants, with 12.2% of the patients having a daily intake ≤500 mg, 22.4% between 501 and 800 mg, 18.9% between 801 and 1000 mg, 33.6% between 1001 and 1500 mg, and 12.8% of the patients with intake >1500 mg.
Subgroup analysis
After excluding patients who began taking any treatment described in the exclusion criteria section after study initiation, lumbar BMD results in the remaining population (n = 672) showed a mean (SD) T-score increase of 0.09 (0.79) in the OHC arm (n = 224) and a decrease of −0.23 (0.70) in the CC group (n = 76) by study end (p < 0.001 for between groups differences). The mean (SD) change for this subgroup was 0.01 (0.11) g/cm2 in the OHC arm, which represented an increase of 0.96%, compared to a mean change of −0.03 (0.10) g/cm2, or a decrease of −3.1%, in the CC group (p < 0.001 for difference between arms).
BMD results were similar when the analyses were re-run in compliant patients (n = 487), with OHC patients (n = 281) showing a mean (SD) increase in lumbar T-score of 0.03 (0.80) and CC patients (n = 101) showing a mean decrease of −0.27 (0.72) (p < 0.0001 between groups). The mean (SD) change in compliant patients was 0.00 (0.11) g/cm2, or no change, in the OHC arm and a decrease of −0.03 (0.10) g/cm2 in the CC group, representing a change of −3.1% (p < 0.001 for the between-group difference).
Finally, in compliant patients who took no drugs which could potentially affect BMD other than the study drugs (n = 361), by study end there was a mean (SD) change in lumbar T-score of 0.10 (0.79) in the OHC arm (n = 214) and a change of −0.27 (0.69) in the CC arm (n = 71). The difference in the size of the change between groups was statistically significant at p < 0.0001. In the same population, the mean (SD) change was 0.01 (0.11) g/cm2 in the OHC arm, which represented an increase of 0.96%, compared to −0.03 (0.10) g/cm2 in the CC group, which represented a change of −3.1% (p < 0.001 for the difference between groups).
Patients with osteoporosis at study end
In patients who took no drugs which could potentially affect BMD except for the study drugs (n = 672), the BMD test results indicated the presence of osteoporosis in 0.8% (n = 4) of the OHC group and in 3.0% (n = 5) of the CC arm (p < 0.05 for the between-group difference).
Similar results were observed in the same group when only compliant patients were evaluated (n = 361), with 1.5% (n = 4) in the OHC arm and 5.6% (n = 5) in the CC group showing osteoporosis (p < 0.05 for the difference between groups).
Reported fractures by treatment group
No clinical vertebral fractures were reported during follow-up. However, 19 patients (2.3%) suffered a bone fracture which, in most cases, was assumed to be caused by high-impact trauma. The rate was higher in the CC group at 3.7% (n = 8), compared to 1.8% (n = 11) in the OHC group, although the difference was not statistically significant.
Tolerability
A total of 34 patients reported at least one ADR (see ) (n = 17 in each group, or 2.7% and 7.7% for the OHC and CC group, respectively; p = 0.001). ADRs were considered mild. A total of 10 patients withdrew from the study because of ADRs; two (0.3%) in the OHC group and eight (3.6%) in the CC group (p < 0.001).
Table 3. Reported adverse drug reactions.
The majority of ADRs were gastrointestinal: 13 patients (2.1%) in the OHC group and 13 (5.9%) in the CC group reported gastrointestinal ADRs at some point in the study (p < 0.005). No cardiovascular events were reported.
Discussion
To our knowledge, this is the first study to comparatively evaluate the long-term efficacy, safety, and tolerability of OHC versus CC for bone loss prevention during perimenopause.
Lumbar BMD was maintained in patients treated with OHC but decreased significantly (3.1%) over the 3-year follow-up period in patients treated with CC.
The results observed here are similar to those reported in previous studies comparing OHC and CC. In a randomized, open-label, 2-year follow-up study carried out in non-osteoporotic postmenopausal womenCitation17, patients treated with OHC maintained their BMD while patients treated with CC had a 3.7% BMD loss by the study end. Similar trends were observed in trials comparing the two drugs in postmenopausal women withCitation18 or withoutCitation15 bone fractures and in elderly patients with osteoporosisCitation14,Citation19. In a meta-analysis of randomized controlled trials which compared OHC and CC, OHC proved to be substantially more effective in preventing bone loss than CCCitation13.
The difference in efficacy between the two calcium supplements does not seem to be related exclusively to the dose of calcium supplementation, as the amount of calcium provided by CC was 40.4% higher that provided by OHC. The difference in efficacy might be explained by the osteogenic effects associated with OHC’s organic component, ossein, as suggested by several authorsCitation11,Citation12,Citation15,Citation20.
Proteins present in OHC (osteocalcin, insulin growth factor type I, insulin growth factor type II, transforming growth factor-β) are considered mitogenic for bone cells in vitroCitation21–23 and could improve bone formation in vivo as observed in different studiesCitation11,Citation12,Citation14,Citation15, although the process is not completely understood.
The osteogenic hypothesis seems to be confirmed by the results of a randomized controlled trial which compared the effects of OHC and CC on bone metabolism in women with osteoporosis aged >65 years without prevalent fracturesCitation14. After 3 years of treatment, it was observed that mean levels of serum osteocalcin significantly increased in patients treated with OHC in comparison with those receiving CC, indicating a greater anabolic effect of OHC on bone.
Both treatments were well tolerated, although OHC appeared to be better tolerated than CC and there was a lower rate of withdrawals due to ADRs in OHC patients. The rates of ADRs observed are similar to those reported in a 4-year follow-up study which evaluated the role of OHC in the prevention of bone lossCitation24. The presence of ADRs is important as this can affect treatment adherence, as reported in a study which showed a statistically significant association between the incidence of adverse effects and reduced adherence to calcium and vitamin D supplementation in patients attending centers specializing in osteoporosisCitation25.
Limitations and strengths
Limitations of this study include the fact that treatments were non-randomized. Although randomized controlled trials are considered the gold standard for evaluating the effectiveness of medical interventions, observational studies have strengths as well, particularly when the aim is to investigate efficacy and safety in conditions of real-world practice. The risk of bias in the study was reduced by the fact that there were no significant differences between the two study groups at baseline and treatment compliance between both groups was similar, ensuring comparability. Further strengths of the study included the long-term follow-up and the fact that this is the largest sample to date in which the long-term safety and efficacy of OHC and CC have been compared in a population of perimenopausal women.
A dropout rate of 57.7% could also be considered a limitation of the study, although those levels of dropout are frequently observed in clinical practiceCitation26–28. The majority of the dropouts were due to loss to follow-up, although adverse effects and concomitant diseases were also reported. Nevertheless, the final sample was sufficiently large to detect statistically significant differences between study groups on the outcomes of interest.
Finally, as the study was intended to assess efficacy and tolerability in real-world clinical practice, the DXA tests were carried out in health centers located in the cities where the patients lived rather than in a sole location. This could have led to slight differences in the coefficient of variation between centers due to the use of different bone densitometers. Nevertheless, this is likely to have had only a limited influence on the results and any potential bias due to the use of different densitometers across centers can also be assumed to affect both groups equally. It is also relevant to note that the 3.1% reduction in lumbar BMD in the CC group in comparison with the OHC group is likely to be clinically relevant, as it is greater than the 2.6% difference observed in the MORE study between raloxifene and placebo, which was shown to be associated with increased risk of vertebral fracture in the placebo groupCitation29.
Conclusions
This study has shown that OHC appears to be significantly more effective than CC at preventing bone loss in the perimenopausal period. Both drugs had a good long-term safety profile although OHC appeared to be better tolerated than CC, especially as regards gastrointestinal events. Given that the calcium dose was approximately 40% higher in the CC group, the superior efficacy of OHC appears to be connected with its ossein constituent. Further research is warranted to confirm the results observed in this study.
Ethical statement
All study participants gave informed consent to participate in this research. The study was conducted in accordance with the ethical standards set forth in the Helsinki Declaration (1983). The study was approved by the Spanish Health Authorities.
Potential conflict of interest
C.C.-B. has disclosed that he has been a recipient of research/grant funding from, has been a consultant/advisor to, and/or has been a lecturer for Gebro, Gedeon Richter, Isdin, Kern, Lacer, Pierre Fabre, Schering Plough, and Shionogi. S.P. has disclosed that he has been a recipient of research/grant funding from, has been a consultant/advisor to, and/or has been a lecturer for Amgen, Bayer-Schering, Exeltis, Gedeon Ritcher, MSD, Novo Nordisk, Pfizer, Pierre Fabre, Procare Health Serelys, Servier, Shionogi, and Teva. J.M. is a medical advisor with Pierre Fabre Ibérica S.A., a company that commercializes an OHC. The other authors do not declare conflicts of interest.
Acknowledgements
The authors thank Michael Herdman for technical assistance and for translating and reviewing the manuscript, Isaac Subirana for his help with statistical analysis, and Pilar Domec for her enormous support throughout the study.
Additional information
Funding
References
- Consensus Development Conference. Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 1993;94:646–50
- Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002;359:1761–7
- Balk EM, Adam GP, Langberg VN, et al. Global dietary calcium intake among adults: a systematic review. Osteoporos Int 2017;28:3315–24
- Seifert-Klauss V, Fillenberg S, Schneider H, Luppa P, Mueller D, Kiechle M. Bone loss in premenopausal, perimenopausal and postmenopausal women: results of a prospective observational study over 9 years. Climacteric 2012;15:433–40
- Chapurlat RD, Garnero P, Sornay-Rendu E, Arlot ME, Claustrat B, Delmas PD. Longitudinal study of bone loss in pre- and perimenopausal women: evidence for bone loss in perimenopausal women. Osteoporos Int 2000;11:493–8
- Lo JC, Burnett-Bowie SA, Finkelstein JS. Bone and the perimenopause. Obstet Gynecol Clin North Am 2011;38:503–17
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014;25:2359–81
- Compston J, Cooper A, Cooper C, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 2017;12:43
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an endocrine society* clinical practice guideline. J Clin Endocrinol Metab 2019;104:1595–622
- Shea B, Wells G, Cranney A, et al. Calcium supplementation on bone loss in postmenopausal women. Cochrane Database Syst Rev 2004;1:CD004526
- Schmidt KH, Worner UM, Buck HJ. Examination of new bone growth on aluminium oxide implant contact surfaces after oral administration of ossein-hydroxyapatite compound to rats. Curr Med Res Opin 1988;11:107–15
- Annefeld M, Caviezel R, Schacht E, Schicketanz KH. The influence of ossein-hydroxyapatite compound (‘Ossopan’) on the healing of a bone defect. Curr Med Res Opin 1986;10:241–50
- Castelo-Branco C, Ciria-Recasens M, Cancelo-Hidalgo MJ, et al. Efficacy of ossein-hydroxyapatite complex compared with calcium carbonate to prevent bone loss: a meta-analysis. Menopause 2009;16:984–91
- Ciria-Recasens M, Blanch-Rubió J, Coll-Batet M, et al. Comparison of the effects of ossein-hydroxyapatite complex and calcium carbonate on bone metabolism in women with senile osteoporosis: a randomized, open-label, parallel-group, controlled, prospective study. Clin Drug Investig 2011;31:817–24
- Pelayo I, Haya J, De la Cruz JJ, et al. Raloxifene plus ossein-hydroxyapatite compound versus raloxifene plus calcium carbonate to control bone loss in postmenopausal women: a randomized trial. Menopause 2008;15:1132–8
- Durance RA, Parsons V, Atkins CJ, Hamilton EBD, Davies C. A trial of calcium supplements (Ossopan) and ashed bone. Clin Trial J 1973;10:67–74
- Castelo-Branco C, Pons F, Vicente JJ, Sanjuán A, Vanrell JA. Preventing postmenopausal bone loss with ossein-hydroxyapatite compounds. Results of a two-year, prospective trial. J Reprod Med 1999;44:601–5
- Rüegsegger P, Keller A, Dambacher MA. Comparison of the treatment effects of ossein-hydroxyapatite compound and calcium carbonate in osteoporotic females. Osteoporosis Int 1995;5:30–4
- Chevalley T, Rizzoli R, Nydegger V, Slosman D, Rapin CH, Michel JP. Effects of calcium supplements on femoral bone mineral density and vertebral fracture rate in vitamin-D-replete elderly patients. Osteoporosis Int 1994;4:245–52
- Castelo-Branco C, Dávila Guardia J. Use of ossein-hydroxyapatite complex in the prevention of bone loss: a review. Climacteric 2015;18:29–37
- Stèpán JJ, Mohan S, Jennings JC, Wergedal JE, Taylor AK, Baylink DJ. Quantitation of growth factors in ossein-mineral-compound. Life Sci 1991;49:79–84
- Saadeh PB, Mehrara BJ, Steinbrech DS, et al. Transforming growth factor-ß1 modulates the expression of vascular endothelial growth factor by osteoblasts. Am J Physiol 1999;277:628–37
- Bonjour JP, Ammann P, Chevalley T, Rizzoli R. Protein intake and bone growth. Can J Appl Physiol 2001;26:153–66
- Fernández-Pareja A, Hernández-Blanco E, Pérez-Maceda JM, Riera Rubio VJ, Palazuelos JH, Dalmau JM. Prevention of osteoporosis: four-year follow-up of a cohort of postmenopausal women treated with an ossein-hydroxyapatite compound. Clin Drug Investig 2007;27:227–32
- Conti F, Piscitelli P, Italiano G, et al. Adherence to calcium and vitamin D supplementations: results from the ADVICE Survey. Clin Cases Miner Bone Metab 2012;9:157–60
- Carnevale V, Nieddu L, Romagnoli E, et al. Osteoporosis intervention in ambulatory patients with previous hip fracture: a multicentric, nationwide Italian survey. Osteoporos Int 2006;17:478–83
- Castelo-Branco C, Cortés X, Ferrer M. UNICAD study investigators. Treatment persistence and compliance with a combination of calcium and vitamin D. Climacteric 2010;13:578–84
- Pfister AK, Welch CA, WuLu JT, Jr, Hager KA, Saville PD. An assessment of postmenopausal women’s adherence to calcium with vitamin D supplements. J Appl Res 2008;8:143–50
- Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999;282:637–45
Appendix
The following investigators are part of the PROP Study Group:
A Coruña: Devesa Hermida, R.; Alicante: Abad Olmos, J.; Amat Sirvent, R.; López Molina, J.B.; Villarroya Navarro, E.; Badajoz: Álamos Carrión, A.; Barcelona: Albert Casali, C.; Bernard Julián, A.; Beroiz Fernández, P.; Feriche Adell, M.; Garrido Saldaña, A.; Saceda López, M.R.; Bilbao: Apodaca Santiesteban, L.A.; Cantabria: Ceballos Rodríguez, M.C.; Castellón: Goberna Burguera, J.; Vilar Igual, M.; Guadalajara: Abollado Fernández, J.L.; Las Palmas: Latorre Alcazo, M.C.; Sosa Marrero, M.; León: Carriles Sastre, R.; Lugo: Fandiño García, M.D.; Madrid: De Castro Martínez, P.; De la Calle Fernández-Miranda, M.; Martín Díaz, V.; Martín Escanciano, F.J.; Muñoz Fernández, M.T.; Pelayo Delgado, I.; Pérez Maceda, J.M.; Piernas Morales, C.; Pino Villalba, M.P.; Prieto Amorín, A.I.; Seco del Cacho, C.; Murcia: Castro Díez, M.C.; Tarragona: Monegal Espinosa de los Monteros, R.; Valencia: Abad Carrascosa, A.; Bueso Casasus, M.J.; Cervera Sánchez, J.; De Gonzalo Santos, A.; Flor Civera, M.F.; García Loscos, J.M.; Marí Sánchez, M.F.; Negueroles Albuixech, R.; Pérez Garcilaso, J.; Ródenas Palazón, J.J.; Zamora: García González, C.E.; Zaragoza: Camo Alcober, F.J.; Díaz Vega, M.; Elorriaga Azpilicueta, C.