Abstract
A systematic literature search revealed 35 clinical studies and one meta-analysis comprising 43,759 women, of which 13,096 were treated with isopropanolic Cimicifuga racemosa extract (iCR). Compared to placebo, iCR was significantly superior for treating neurovegetative and psychological menopausal symptoms, with a standardized mean difference of −0.694 in favor of iCR (p < 0.0001). Effect sizes were larger when higher dosages of iCR as monotherapy or in combination with St. John’s wort (Hypericum perforatum [HP]) were given (−1.020 and −0.999, respectively), suggesting a dose-dependency. For psychological symptoms, the iCR+HP combination was superior to iCR monotherapy. Efficacy of iCR was comparable to low-dose transdermal estradiol or tibolone. Yet, due to its better tolerability, iCR had a significantly better benefit–risk profile than tibolone. Treatment with iCR/iCR+HP was well tolerated with few minor adverse events, with a frequency comparable to placebo. The clinical data did not reveal any evidence of hepatotoxicity. Hormone levels remained unchanged and estrogen-sensitive tissues (e.g. breast, endometrium) were unaffected by iCR treatment. As benefits clearly outweigh risks, iCR/iCR+HP should be recommended as an evidence-based treatment option for natural climacteric symptoms. With its good safety profile in general and at estrogen-sensitive organs, iCR as a non-hormonal herbal therapy can also be used in patients with hormone-dependent diseases who suffer from iatrogenic climacteric symptoms.
摘要
一项系统的文献搜索显示, 35项临床研究和一项meta分析涉及43,759名女性, 其中13,096人接受了异丙醇总状升麻提取物(iCR)的治疗。与安慰剂相比, iCR在治疗神经植物性和心理绝经症状方面明显优于安慰剂, 标准化均数差为-0.694(p<0.0001)。当更大剂量的iCR作为单一治疗或与贯叶连翘(金丝桃 [HP])合用时, 其效应大小更大(分别为-1.020和-0.999), 表明存在剂量依赖关系。然而, 由于其更好的耐受性, iCR的益处-风险情况比替勃龙更好。iCR/iCR+HP治疗耐受性良好, 几乎没有轻微不良反应, 频率与安慰剂相当。临床数据没有显示任何肝毒性的证据。激素水平保持不变, 雌激素敏感组织(如乳腺、子宫内膜)不受iCR治疗的影响。由于益处明显大于风险, iCR/iCR+HP应被推荐为自然绝经症状的循证治疗选择。iCR作为一种非激素的草药疗法, 具有良好的安全性和对雌激素敏感的器官, 也可用于有医源性绝经症状的激素依赖型疾病患者。
Introduction
Up to 80% of climacteric women, especially those suffering from hot flushes, use complementary and alternative medicine, mainly without informing their health-care providersCitation1–5. This highlights not only patients’ wishes but also a need for evidence-based information on the efficacy and safety of these treatments.
One of the most popular herbal remedies is Cimicifuga racemosa (CR) syn. Actaea racemosa (black cohosh). CR has been used by Native Americans and eclectic physicians as a gynecological remedy and for rheumatism and other conditions. The earliest literature references date back to the seventeenth centuryCitation6. The first allopathic herbal medicinal product (HMP) containing CR rootstock extract was introduced in Germany in 1956 and has been extensively studied sinceCitation7. In Germany, where HMPs are authorized medicines under strict regulatory control requiring state-of-the-art proof of efficacy, safety, and pharmaceutical quality, gynecologists rate CR treatment as well-known and effective for climacteric symptomsCitation8. In many other countries, CR products are marketed as food supplements (FS). FS are not as rigorously controlled as HMPs and run the risk of adulteration and contamination. Frequently, FS claiming to consist of authentic North American CR have contained completely different Asian Cimicifuga speciesCitation9. While FS often have little or no scientific evidence behind them, they are regarded as complementary and alternative medicine. On the other hand, (CR) HMPs are evidence-based rational phytopharmaceuticals and should be treated as suchCitation10.
In 2011, and again in 2018, the European Medicines Agency issued an official monograph with a positive benefit–risk ratio for CR HMPs for the treatment of climacteric symptomsCitation11,Citation12. A Cochrane review doubted CR’s superiority versus placebo for menopausal symptomsCitation13 but was internationally criticized soon after. Apart from a failure to identify all appropriate randomized controlled trials (RCTs) and an incorrect exclusion of positive results, the review’s main shortcoming was a missing differentiation between the extracts usedCitation14. The new meta-analysis showed CR to be significantly superior to placebo for menopausal symptomsCitation14.
Unlike synthetic drugs, the active ingredient of phytopharmaceuticals is not a single substance but the whole plant extract. Herbal extracts contain a multitude of different constituents and – even if derived from the same plant – their composition varies depending on many factors (e.g. cultivation, harvesting conditions, extraction process, extractant, and standardization) that may lead to different effects and efficaciesCitation15. Therefore, study results gained with a specific extract cannot be transferred one-to-one to different extracts of this herb. For phytopharmaceuticals there are no genuine generics as with synthetic drugs. Consequently, when analyzing scientific data, it is crucial to differentiate between HMPs and non-HMPs/FS and between different extracts. The first differentiated evaluation of extract-specific evidence on CR’s efficacy and safety was performed by Beer and Neff in 2013Citation16. Their analysis of publications from 2000 to 2012 came to the conclusion that only HMPs, but not FS/non-HMPs, of CR demonstrated evidence for their efficacy. Best evidence was provided by the isopropanolic CR extract (iCR); the only extract tested in several thousand women and the only extract that provided confirmatory Level 1 evidence and Grade of Recommendation ACitation16. These results were confirmed by an additional analysis of newer studies published from 2012 to 2014Citation17.
This review’s purpose is to give a current update and overview of all placebo-controlled clinical data (irrespective of publication date) and additional data from clinical studies with iCR during a broader time span ranging from the establishment of the EU Guideline on Good Clinical Practice E6 in 1997 until January 2020. Furthermore, for the first time, all placebo-controlled data gained with iCR are pooled in a meta-analysis. Efficacy and safety results are analyzed in detail and discussed in order to enable health-care providers to advise patients who refuse or have contraindications to hormone therapy for climacteric symptoms.
Methods
MEDLINE, EMBASE, EMBASE Alert, BIOSIS, and PubMed were searched for clinical studies with iCR (irrespective of design) and meta-analyses thereof, published from 1997 to January 2020, as a basis for our review. Furthermore, all available randomized placebo-controlled data, irrespective of publication date, were collected as a basis for our meta-analysis. The database search was complemented by a manual search in the authors’ library and an additional search in ClinicalTrials.gov and the EU Clinical Trials Register.
Inclusion criteria were medical use of iCR, efficacy for neurovegetative and psychological climacteric symptoms, additional clinical benefits, occurrence and frequency of adverse events, and influence on liver, hormones, and estrogen-sensitive organs. No restrictions regarding patients’ ages, menopausal status, and treatment duration were made. Patient-relevant endpoints were reduction of neurovegetative and psychological climacteric symptoms (e.g. scales for climacteric symptoms, symptom frequency and intensity, and objective symptom measurement), additional clinical benefits, and frequency and severity of adverse events, including influence on estrogen-sensitive organs or laboratory parameters.
Studies were sorted according to their quality and the RCTs regarding their control group (placebo, active comparator). The placebo-controlled RCTs were pooled in the meta-analysis and independently assessed for risk of bias with the revised Cochrane RoB 2 tool (2019) by three authors (M.G., H.H.H.-v.Z., and S.P.). Disagreements were discussed until a consensus was reached. The meta-analysis was performed using SAS version 9.4 under the fixed-effect size model. For studies that did not directly report the standardized group difference and the corresponding confidence interval, these parameters were deduced either from the published means, standard deviations, and sample size N or from the published means, sample size N, and p-values.
Results
The search revealed one meta-analysisCitation18 and 35 clinical studiesCitation19–53, including 16 RCTsCitation19–31,Citation50,Citation52,Citation53, that met the inclusion criteria. Altogether, 43,759 patients were included in the studies; 13,096 of the patients were treated with iCR. A detailed overview of populations, interventions, and results for each study is presented in Table S1.
Table 1. Characteristics of the double-blind RCTs comparing iCR with placebo for neurovegetative and psychological menopausal symptoms, used as the basis for the meta-analysis.
Efficacy for natural menopausal symptoms
Placebo-controlled, double-blind RCTs
The first RCT demonstrated that iCR was superior to placebo in improving the Kupperman Menopause Index (KMI) and the Hamilton Anxiety Rating ScaleCitation19 scores for all assessment points (4, 8, and 12 weeks; p < 0.001). In the iCR group, the average symptom intensity decreased from ‘moderate’ at baseline to ‘mild’ at study termination, showing a good therapeutic result. In the placebo group, it decreased but still remained ‘moderate’.
Significantly greater improvements in the total Menopause Rating Scale (MRS) scores were found in patients taking iCR for 3 months compared to placebo (p < 0.001)Citation22. Efficacy was best for vasomotor symptoms (hot flushes, sweating, and sleep disorders). With 0.03–0.05 MRS units, the effect size was similar to that of 0.6 mg conjugated estrogens in a previous study and clinically relevant. The therapeutic benefit was highest in patients who were treated in the early climacteric phase.
In iCR-treated patients, significantly higher reductions in climacteric symptom severity (total KMI and hot flush scores) were seen after 4 weeks (p < 0.05 for KMI, p = 0.041 for hot flushes) and 12 weeks (p < 0.001 for KMI, p = 0.021 for hot flushes) compared to placeboCitation26. iCR noticeably decreased both scores from baseline to study termination (KMI, 26.46 ± 10.46 to 6.37 ± 4.16; hot flushes, 7.52 ± 2.37 to 1.52 ± 1.97).
Polysomnography, conducted to objectively measure sleep disturbances in a 6-month trial, showed that patients using iCR had significantly increased sleep efficiency (p = 0.01) and decreased wake after sleep onset duration (p < 0.01) compared to placebo. Vasomotor and physical domains of the Menopause-specific Quality of Life questionnaire also improved versus placeboCitation31.
The fixed combination of high-dose iCR plus low-dose St. John’s wort (Hypericum perforatum) (iCR+HP) was significantly superior to placebo in improving MRS (−50%) and Hamilton Depression Rating Scale scores (−41.8%) (p < 0.001)Citation24.
Active-controlled RCTs
A 3-month study compared the efficacy of iCR to low-dose transdermal estradiol for climacteric symptomsCitation23. Both treatments significantly reduced the number of hot flushes/day and vasomotor symptoms as well as anxiety and depression (p < 0.001 for all). Both treatments showed comparable efficacy without significant differences.
Chinese women with climacteric symptoms received either iCR or tibolone for 3 months in a double-blind, double-dummy trialCitation25. A noticeable and clinically relevant reduction of similar size for the KMI scores was seen in both groups, significantly demonstrating non-inferiority of iCR (p = 0.002). Yet, due to its better tolerability, iCR was superior to tibolone in the benefit–risk ratio (p = 0.01).
In two further RCTs, significant KMI score reductions of perimenopausal symptoms were shown under iCR after 3 months; they were comparable to those under tiboloneCitation52,Citation53. Additionally, vaginal atrophy symptoms were significantly ameliorated in both groups, without significant group differencesCitation53.
Treatment with a standard dose (SD) or high dose (HD) of iCR for 6 months significantly decreased menopausal symptoms (KMI score reduced by 75%). The responder rate was already 70% after 3 months. These effects were maintained after 6 monthsCitation21. For the complete study population, no differences between SD and HD could be observed. However, patients who were in the early climacteric phase benefited significantly more from HD compared to SD and also exhibited a response rate above 90% when using HDCitation21,Citation54.
Women with perimenopausal depression were treated with either paroxetine or paroxetine plus iCR for 2 monthsCitation28. The iCR group showed significantly better improvement in the Hamilton Depression Rating Scale (p < 0.05) and KMI (p < 0.01) scores compared to patients taking only paroxetine.
A 12-week iCR treatment was compared to placebo treatment in patients with postmenopausal atrophic vaginitisCitation27. While atrophic vaginitis symptoms were significantly reduced in the iCR group (p < 0.05), there were no significant influences on vaginal pH or the maturation degree of vaginal epithelial cells.
Open, controlled studies
In a prospective, controlled observational study, 6,141 patients were treated for 6–12 months (n = 736) with iCR or iCR+HP according to the physicians’ choicesCitation32. Both treatments led to significant reductions of the MRS total score and the MRS subscore ‘psyche’. The iCR+HP combination was superior to the monotherapy in ameliorating psychological climacteric symptoms (p < 0.001).
Compared to untreated control, iCR-treated women showed significant decreases in the urinary concentration of N-telopeptides (bone resorption marker) and increases in serum alkaline phosphatase (bone formation marker) after 3 monthsCitation33.
Open, uncontrolled studies
Two studies using a 3-month iCR therapy found significant reductions in the KMI scores. The most pronounced improvements were seen for hot flushes and sweatingCitation42,Citation43. Sleeping difficulties and anxiety were also greatly reducedCitation43. Postmenopausal women with increased body weight taking iCR for 3 months experienced a significant increase in their quality of life (Cervantes Health-Related Quality-of-Life Scale), especially in the ‘menopause/health’ and ‘psyche’ domainsCitation47. Another 3-month study found that more than 70% of women using iCR had fewer hot flushes, less sweating, and fewer psychological complaints (irritability, depressive moods, and insomnia)Citation45. Patients taking HD iCR for 98 days exhibited reductions in 10 typical neurovegetative climacteric symptoms. Efficacy was rated as good or very good in 80% of the casesCitation40. The combination of iCR+HP ameliorated not only psychological but also neurovegetative symptoms of menopause significantly after 12 weeks of therapy. Efficacy was judged as good or very good in 81% of the casesCitation39.
Efficacy for iatrogenic menopausal symptoms
Placebo-controlled, double-blind RCTs
Breast cancer patients, most treated with tamoxifen, received either iCR or placebo for 2 months. While both groups had a comparable decline in the number and intensity of hot flushes, the iCR-treated women reported significantly less sweating than the placebo group (p = 0.04)Citation20.
Active-controlled RCTs
Endometriosis patients were treated with postoperative goserelin therapy, causing estrogen-deficiency symptomsCitation30. Four weeks after the first goserelin injection, they received add-back therapy with tibolone or iCR for 12 weeks. Both treatments significantly reduced KMI and hot flushes/sweating scores to a comparable extent without significant group differences. The iCR group had significantly fewer adverse events and less vaginal bleeding/spotting or breast pain.
Open, controlled studies
Women with early breast cancer were treated with luteinizing hormone-releasing hormone analogue after surgery, mostly combined with endocrine therapy. They randomly received iCR or no treatment for 3 months. The iCR group had significantly lower KMI scores compared to controlCitation50.
Patients with stage 1 endometrial cancer suffering from menopausal symptoms after surgery were treated with iCR for 24 weeks or remained untreated as controlCitation35. All patients were followed for 12 months. Therapy with iCR resulted in a significant decrease of the KMI score and a significant increase in bone mineral density (both p < 0.05).
Open, uncontrolled studies
A pilot trial in women (breast cancer, high risk, or refused hormone therapy) with significant hot flushes, partly caused by tamoxifen or raloxifene, found a reduction of hot flush frequency (−50%), severity (−22%), and sweating (−80%) after 4 weeks of iCR intakeCitation41. Additionally, patients reported less sleeping problems and less fatigue.
Patients after surgery for gynecological cancers (endometrial, ovarian, cervix, borderline ovarian, and breast cancers) experienced a significant reduction of KMI scores, hot flushes, sweating, and depressive moods after 3 months of treatment with iCR (p < 0.05)Citation48.
Women after breast cancer surgery suffering from tamoxifen-induced climacteric symptoms were treated with iCR for 6 months. The dosage was adapted to symptom intensityCitation49. The iCR treatment led to significant reductions in the total MRS II score (p < 0.001). Hot flushes, sweating, sleep problems, and anxiety improved most.
Meta-analysis of the placebo-controlled RCTs
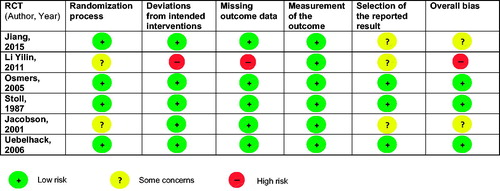
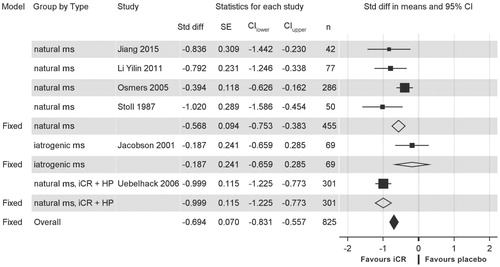
All placebo-controlled RCTs examining efficacy for neurovegetative and psychological climacteric symptoms were included in the meta-analysisCitation19,Citation20,Citation22,Citation24,Citation26,Citation31. Most of the RCTs had a low risk of biasCitation19,Citation22,Citation24. Two RCTs raised some concernsCitation20,Citation31 and one RCT was rated as high risk according to RoB 2 criteriaCitation26 (; extensive assessment presented in Table S2). However, the latter suffered explicitly from low-quality reporting, which may not necessarily imply an actual risk of bias. Detailed characteristics of these RCTs are presented in . shows a forest plot of these RCTs, revealing an overall standardized mean difference of −0.694 in favor of iCR (p < 0.0001). By subgroups, the standardized mean difference in favor of iCR was −0.568 for natural menopausal symptoms, −0.187 for iatrogenic symptoms, and −0.999 in favor of iCR+HP for natural climacteric symptoms. For studies with higher iCR dosages as monotherapy or in combination with HPCitation19,Citation24, the standardized mean difference in favor of iCR was −1.020 and −0.999, respectively.
Figure 2. Forest plot of isopropanolic Cimicifuga racemosa extract (iCR) versus placebo in neurovegetative and psychological menopausal symptoms.
CI, confidence interval; Fixed, summary of the respective group of studies under the fixed-effect size model; HP, Hypericum perforatum (St. John’s wort); ms, menopausal symptoms; n, number of patients; Std diff, standardized mean difference; SE, standard error of the standardized difference.
Safety in general and at the liver
Treatment with iCR was generally well tolerated with few minor adverse eventsCitation19,Citation21–23,Citation25,Citation30,Citation32,Citation33,Citation39,Citation41,Citation43–45,Citation52 or even no adverse eventsCitation27,Citation31,Citation35. Compared to placebo, the frequency of adverse events under iCR or iCR+HP was not significantly differentCitation22,Citation24. HD iCR demonstrated the same good tolerability as SD iCRCitation21. In breast cancer patients treated with tamoxifen, typical tamoxifen-associated adverse effects occuredCitation20,Citation49. Compared to tibolone, iCR treatment had significantly fewer adverse effects, especially for vaginal bleeding or spottingCitation25,Citation30,Citation52,Citation53. No significant influences of iCR on body weight/body mass indexCitation22,Citation25,Citation35, heart rateCitation22, blood pressureCitation22,Citation28,Citation35, or electrocardiogramCitation28 were seen. Routine hematologyCitation21,Citation24,Citation25,Citation28,Citation48, routine biochemistryCitation21,Citation24,Citation25,Citation33,Citation48, including kidney function testsCitation21,Citation24,Citation25,Citation28,Citation30,Citation31,Citation35,Citation48,Citation52,Citation53, and urinalysisCitation25,Citation28,Citation48 remained unaffected by iCR. Regarding lipid metabolism, total cholesterol and triglycerides were not affectedCitation23,Citation26,Citation30,Citation33,Citation35,Citation44,Citation48,Citation52,Citation53. However, several investigators reported significant increases of high-density lipoprotein cholesterol and decreases of low-density lipoprotein cholesterolCitation23,Citation26,Citation48,Citation52.
A meta-analysis of five double-blind RCTs including 1117 patients showed that SD or HD iCR treatment for 3–6 months did not significantly influence liver function tests and did not reveal any evidence for hepatotoxicityCitation18. Ten further studiesCitation23,Citation26,Citation28,Citation30,Citation31,Citation35,Citation48,Citation52,Citation53,Citation55, seven of which were RCTs, including 741 patients also did not find any significant changes in liver function parameters.
Safety at estrogen-sensitive organs
Taking SD iCR for 3–6 months did not lead to significant changes in gonadotropin or estradiol levels in otherwise healthy women (n = 481)Citation21,Citation23,Citation26,Citation27,Citation31,Citation33,Citation40,Citation46,Citation52,Citation53 or in women with estrogen-dependent diseases (breast cancer, gynecological tumors, and endometriosis) (n = 193)Citation20,Citation30,Citation35,Citation48,Citation50. HD iCR also did not affect follicle stimulating hormone, luteinizing hormone, or estradiolCitation21,Citation40. Furthermore, prolactin, sex hormone binding globulin, and testosterone remained unchangedCitation21,Citation23,Citation33,Citation40,Citation44.
Ultrasound scans showed that endometrial thickness was not influenced by 3–6-month iCR treatment (n = 516)Citation23,Citation25–27,Citation31,Citation40,Citation44,Citation50,Citation52,Citation53. Also, in patients with uterine pathologies like endometriosis or endometrial carcinoma, pelvic ultrasound was unremarkable (n = 86)Citation30,Citation35. iCR therapy in goserelin-treated endometriosis patients resulted in significantly less vaginal bleeding/spotting than add-back therapy with tiboloneCitation30. Using iCR for 3 months led to a significant reduction of myoma volume compared to baseline (−30%) and compared to tibolone, which had no significant effects (+4.7%)Citation29. Patients with endometrial cancer had no increase of tumor marker CA 125 or recurrences during 6-month iCR treatment and 12-month follow-upCitation35. No significant changes of vaginal cytology/maturation index or pH could be detected after 3–6-month treatment with SD or HD iCRCitation21,Citation26,Citation27,Citation40.
Breast ultrasonography did not reveal any changes after 6-month iCR treatment in healthy women and in women after therapy for estrogen-dependent tumors (n = 54)Citation31,Citation35. Furthermore, no signs of increased mammographic breast density (visual assessment) or breast cell proliferation (fine-needle aspiration biopsy) were found after 6-month iCR treatmentCitation44. These results were confirmed by digitized assessment of the mammogramsCitation34. Patients with ductal carcinoma in situ, using iCR for 2–5 weeks pre surgery, experienced no significant changes in proliferation marker Ki67, yet a downward trend was seenCitation51. Two case–control studies demonstrated that iCR use was associated with a reduced risk for breast cancerCitation37,Citation38. The larger study found that risk reduction was independent of lifestyle factors, tumor histology, and receptor status, but improved with longer treatment duration. A pharmacoepidemiological cohort study in breast cancer survivors demonstrated that women taking iCR for climacteric symptoms compared to women not using iCR had a significantly lower risk for recurrence with a 4.5-year longer recurrence-free survival on averageCitation36.
Discussion
All studies with iCR consistently demonstrated its efficacy for natural climacteric symptoms, exhibiting significant superiority versus placebo and an efficacy comparable to low-dose transdermal estradiol or tiboloneCitation19,Citation21–26,Citation28,Citation31,Citation32,Citation39,Citation40,Citation42,Citation43,Citation45,Citation47,Citation52,Citation53. Yet, due to its better tolerability, iCR had a better benefit–risk profile than tibolone. These results confirm the NICE guideline meta-analyses. There, CR was significantly superior to placebo in reducing vasomotor symptom frequency, revealing a good effect size, whereas tibolone was associated with high treatment discontinuationCitation56. It is noteworthy that among all non-hormonal treatments, CR exhibited the largest effect size and had the highest probability of being the best treatmentCitation56. However, the guideline calls for caution concerning the great variety of CR products. This makes sense because inconclusive data on CR’s efficacy result from products that do not meet the strict criteria for HMPsCitation16,Citation17. With a standardized mean difference of −0.568 in favor of iCR versus placebo for natural menopausal symptoms in our meta-analysis, iCR, as an authorized HMP, offers an evidence-based, recommendable, non-hormonal treatment. The inclusion of one publication with a potential for high risk of biasCitation26 in our meta-analysis could be worth discussing. However, as its effect size does not deviate from the overall treatment effect, its inclusion or exclusion has no impact on the result of this meta-analysis. Most of the included studies had a low risk of biasCitation19,Citation22,Citation24.
Our meta-analysis shows higher effect sizes when higher doses of iCR (without or with HP)Citation19,Citation24 were used (standard mean difference of −1.020 and −0.999, respectively), suggesting a dose-dependency. Women in their early climacteric phase may benefit from an increase from 40 mg up to 120 mg drug/dayCitation54. Furthermore, iCR+HP was superior to iCR in ameliorating psychological climacteric symptomsCitation32. With its higher iCR dose and synergistic effects with HP, the combination offers a valuable treatment option not only in terms of its greater effect size shown in our meta-analysis but also because psychological symptoms like depressive moods often occur during perimenopauseCitation57,Citation58.
Efficacy data concerning iatrogenic climacteric symptoms are varied, with the findings being predominantly positive. Climacteric symptoms after endometrial cancer surgery were significantly reduced by iCR over 24 weeks compared to untreated controlCitation35. These positive effects are in line with results of another 24-week, hormone therapy-controlled study with iCR in hysterectomized patientsCitation59. For goserelin-induced symptoms in endometriosis patients, 12 weeks of iCR treatment was as effective as add-back therapy with tibolone, yet more tolerableCitation30. These results were recently confirmed by a study in gonadotropin-releasing hormone analogue-treated breast cancer patients. There, iCR treatment significantly lowered KMI scores after 12 weeks compared to controlCitation50. In tamoxifen-treated breast cancer patients, hot flush reductions over 8 weeks under iCR were not significantly different from placebo, but the sample size was too small to prove superiority; however, iCR-treated patients still benefited from significantly less sweatingCitation20. This study was too short to expect significant efficacy results. The authors assumed that ‘it is possible that when used for a longer period of time, black cohosh may show greater efficacy’. Excluding this study with a shorter treatment duration than the US Food and Drugs Administration and the European Medicines Agency require would have increased the homogeneity of our meta-analysis results. Significant reductions of iatrogenic hot flushes, sweating, and psychological symptoms were shown in longer open studies (12–24 weeks)Citation48,Citation49. There, tamoxifen-treated breast cancer patients were also allowed to double the standard iCR doseCitation49. This could enhance treatment effects, as practical experience showed that patients with tamoxifen-induced symptoms often need higher CR doses than natural menopausal womenCitation60. Thus, HD iCR or iCR+HP can be used to treat climacteric symptoms induced by endocrine therapy. These patients cannot take hormones and without additional therapy may discontinue the anti-hormonal treatment due to its severe side effectsCitation60–62.
Treatment with SD/HD iCR or iCR+HP was well tolerated with only rare minor adverse effects, no significant differences from placebo, and significantly better tolerability compared to tiboloneCitation19,Citation21–25,Citation27,Citation28,Citation30,Citation31,Citation33,Citation35,Citation39–45,Citation48,Citation49,Citation52,Citation53. This favorable safety profile of iCR is congruent with data analyzed for CR extracts in generalCitation13,Citation16,Citation17,Citation63. None of the iCR studies showed liver-specific side effects. Additionally, a meta-analysis of five RCTs and 10 further studies, 1858 patients in total, showed no influence of iCR on liver functionCitation18,Citation23,Citation26,Citation28,Citation30,Citation31,Citation35,Citation48,Citation52,Citation53,Citation55. Single cases of hepatotoxicity had been reported during the use of CR products. These included FS that do not meet the strict quality criteria of HMPs and are often adulterated with different Asian Cimicifuga speciesCitation9. Case report assessment with a liver-specific diagnostic algorithm did not reveal a causal relationshipCitation64–67.
In the past it was believed that CR’s efficacy may be based on estrogen-like effects due to its reputed constituent formononetin. However, this isoflavone was never detected in CR extracts used for the manufacture of iCRCitation68,Citation69. Activity on estrogen receptors was not shown for iCRCitation70. Instead, binding and modulation of central nervous system key receptors for thermoregulation, mood, and sleep (e.g. serotonin, dopamine, γ-aminobutyric acid, µ-opioid) and favorable changes in brain metabolism and activity were observedCitation46,Citation71–75. These mechanisms of action explain not only the efficacy for climacteric symptoms but also the fact that iCR did not influence hormones, breast tissue density or proliferation, endometrial thickness, or vaginal cytologyCitation20,Citation21,Citation23,Citation25–27,Citation30,Citation31,Citation33–35,Citation40,Citation44,Citation48,Citation50,Citation52,Citation53. The amelioration of vaginal atrophy symptoms observed by some researchers can possibly be explained by anti-inflammatory and anti-oxidative effects of CR. The reduction of psychological menopausal symptoms by iCR may also play an important role in this regard, as psychological stress impairs vaginal health and sexual function. Against this background and in line with the official monographs, the benefit–risk ratio of iCR/iCR+HP is clearly positive. The resulting recommendation of iCR for patients with climacteric symptoms is fortified by results of the NICE guideline health economic model. Out of all treatments in hysterectomized women and in women with a uterus, CR had the second highest and the highest probability, respectively, of being the most cost-effective treatmentCitation76.
Furthermore, initial data suggest additional benefits. The longer recurrence-free survival in breast cancer patients taking iCRCitation36 and an association of iCR therapy with a decreased risk of developing breast cancerCitation37,Citation38 are substantiated by experimental data. iCR dose-dependently inhibited the proliferation of estrogen-dependent and estrogen-independent breast cancer cells due to caspase activation and induction of apoptosis; it reduced invasiveness and enhanced the effects of tamoxifenCitation77–81. CR downregulated the expression of estrogen receptor-α, progesterone receptor, and BRCA1 in hormone-dependent breast cancer cells and decreased their proliferationCitation82. In breast cancer animal models, application of iCR indicated a trend toward or actually led to reduced tumor growth – the latter accompanied with prolonged life spanCitation83,Citation84. Combination of tamoxifen with iCR increased the incidence of tumor-free animals and reduced the individual tumor burdenCitation85.
Further mechanisms of action of CR and its constituents may contribute to iCR’s beneficial effects (not only) in breast cancer patients. Actein, a CR triterpene glycoside, had growth inhibitory effects on human breast cancer cells associated with activation of stress response pathwaysCitation86. Actein had anti-angiogenic effects in vitro and decreased breast tumor size and metastasis in the mouse 4T1 breast tumor modelCitation87. Recently, actein was found to suppress human bladder cancer cell proliferation and induce autophagy and apoptosis via activation of reactive oxygen species/c-Jun N-terminal kinase and inhibition of the AKT/mammalian target of rapamycin (mTOR) pathway. The latter is activated in breast and other cancers, leading to tumor cell growth, proliferation, metastasis, and angiogenesis, and is implicated in endocrine resistanceCitation88–90. Additionally, CR and some of its components activated 5′ adenosine monophosphate-activated protein kinase (AMPK) to the same extent as metforminCitation91. AMPK activation and mTOR inhibition suppressed the proliferation and angiogenesis of leiomyoma cells, and mTOR inhibition decreased the tumor incidence, multiplicity, and size in the Eker rat model for myomaCitation92–94. AMPK activation and mTOR inhibition may explain the observed shrinking of myoma under iCR treatment.
Clinical findings suggesting osteoprotective effects of iCR are supported by experimental data: iCR dose-dependently increased osteoprotegerin production (and thereby the osteoprotegerin–RANK ligand ratio) in human osteoblasts, which inhibits osteoclast differentiation and activity and may impede bone resorptionCitation95,Citation96. The osteoblast differentiation markers osteocalcin and alkaline phosphatase activity were increasedCitation95,Citation96. In the ovariectomized rat model for osteoporosis, iCR augmented bone density and qualityCitation97, protected against bone loss, and increased trabecular bone structure and resistanceCitation98. 25-acetylcimigenol xylopyranoside (ACCX), a CR triterpene glycoside, inhibited osteoclastogenesis by abrogation of the nuclear factor-κB and ERK pathways induced by RANK ligand or tumor necrosis factor-αCitation99.
Oxidative stress and inflammatory processes play a significant role not only in menopausal vasomotor symptoms but also in the development and progression of osteoporosis, (breast) cancer, myoma, and further age-related conditions (e.g. cardiovascular disease and cognitive decline)Citation100–103. As iCR exhibited anti-oxidative and mitochondria-protective effectsCitation104 and recent evidence demonstrated strong anti-inflammatory effects for CRCitation105, these effects may contribute to iCR’s efficacy for vasomotor symptom and to possible additional benefits for patients with breast cancer, myoma, or risk for osteoporosis. With numerous mechanisms of action that may explain such benefits, iCR offers an interesting field for future research, especially for diseases with underlying inflammatory, AMPK, or mTOR dysregulation.
Conclusion and practical consequences
In summary, the clinical data and our meta-analysis consistently demonstrate that iCR/iCR+HP is an effective and safe, evidence-based treatment option for natural neurovegetative and psychological climacteric symptoms, meeting increasing patients’ demands for non-hormonal, herbal therapies. As benefits clearly outweigh risks, iCR/iCR+HP should be recommended to these women. With its good safety profile in general and at estrogen-sensitive organs, iCR can also be used in patients with hormone-dependent tumors suffering from iatrogenic menopausal symptoms. However, this should not take place in self-medication but under medical supervision. Breast-cancer patients are not appropriate candidates for non-supervised self-medication, and it seems reasonable that HD iCR or alternatively iCR+HP are more effective in patients with medication-induced symptoms. Breast cancer patients taking iCR may possibly benefit from prolonged recurrence-free survival, which should be confirmed by further clinical trials.
Potential conflict of interest
A.-M. Beer, A. Cano, M. Gambacciani, M. J. Minkin, D. Rachoń, and J. Schnitker report no conflict of interest. C. Castelo-Branco and X. Ruan received fees from Schaper & Brümmer outside the submitted work for lecturing at and chairing, respectively, a congress symposium on iCR in 2019. H.-H. Henneicke-von Zepelin and S. Pickartz are employees of Schaper & Brümmer, the manufacturer of iCR. The authors alone are responsible for the content and writing of this article.
Source of funding
Nil.
Supplemental Material
Download MS Word (48.6 KB)Supplemental Material
Download MS Word (71.8 KB)Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Bair YA, Gold EB, Zhang G, et al. Use of complementary and alternative medicine during the menopause transition: longitudinal results from the Study of Women's Health Across the Nation. Menopause 2008;15:32–43
- Peng W, Adams J, Hickman L, Sibbritt DW. Longitudinal analysis of associations between women's consultations with complementary and alternative medicine practitioners/use of self-prescribed complementary and alternative medicine and menopause-related symptoms, 2007–2010. Menopause 2016;23:74–80
- Peng W, Adams J, Hickman L, Sibbritt DW. Complementary/alternative and conventional medicine use amongst menopausal women: results from the Australian Longitudinal Study on Women's Health. Maturitas 2014;79:340–2
- Buhling K, Daniels B, Studnitz F, Eulenburg C, Mueck A. The use of complementary and alternative medicine by women transitioning through menopause in Germany: results of a survey of women aged 45–60 years. Complement Ther Med 2014;22:94–8
- Posadzki P, Lee MS, Moon TW, Choi TY, Park TY, Ernst E. Prevalence of complementary and alternative medicine (CAM) use by menopausal women: a systematic review of surveys. Maturitas 2013;75:34–43
- Upton R. Black Cohosh Rhizome Actaea racemosa L. syn. Cimicifuga racemosa (L) Nutt Standards of analysis, quality control, and therapeutics American Herbal Pharmacopoeia and Therapeutic Compendium Santa Cruz. CA American Herbal Pharmacopoeia 2002;1–38
- Henneicke-von Zepelin HH. 60 years of Cimicifuga racemosa medicinal products: clinical research milestones, current study findings and current developmnt. Wien Med Wochenschr 2017;167:147–13
- von Studnitz FS, Eulenburg C, Mueck AO, Buhling KJ. The value of complementary and alternative medicine in the treatment of climacteric symptoms: results of a survey among German gynecologists. Complement Ther Med 2013;21:492–5
- Jiang B, Kronenberg F, Nuntanakorn P, Qiu MH, Kennelly EJ. Evaluation of the botanical authenticity and phytochemical profile of black cohosh products by high-performance liquid chromatography with selected ion monitoring liquid chromatography-mass spectrometry. J Agric Food Chem 2006;54:3242–53
- Edwards SE, Da Costa Rocha I, Williamson EM, Heinrich M, Phytopharmacy-An Evidence-Based Guide to Herbal Medicinal Products. Chichester: John Wiley & Sons; 2015
- Community herbal monograph on Cimicifuga racemosa (L.) Nutt., rhizoma [Internet]. 2010. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Community_herbal_monograph/2011/01/WC500100981.pdf
- European Union herbal monograph on Cimicifuga racemosa (L.) Nutt., rhizoma. EMA/HMPC/48745/2017 [Internet]. 2018. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Herbal_monograph/2017/08/WC500233056.pdf
- Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. The Cochrane Library 2012;1–92
- Beer A-M, Osmers R, Schnitker J, Bai W, Mueck AO, Meden H. Efficacy of black cohosh (Cimicifuga racemosa) medicines for treatment of menopausal symptoms – comments on major statements of the Cochrane Collaboration report 2012 "black cohosh (Cimicifuga spp.) for menopausal symptoms (review). Gynecol Endocrinol 2013;29:1022–5
- Schulz V, Hänsel R, Rationale Phytotherapie: Ratgeber für die ärztliche Praxis: Springer-Verlag; 2013
- Beer AM, Neff A. Differentiated evaluation of extract-specific evidence on Cimicifuga racemosa's efficacy and safety for climacteric complaints. Evid Based Complement Alternat Med 2013;2013:860602
- Beer AM. Cimicifuga racemosa bei klimakterischen Beschwerden. Z Phytother 2015;36:10–17
- Naser B, Schnitker J, Minkin MJ, de Arriba SG, Nolte KU, Osmers R. Suspected black cohosh hepatotoxicity: no evidence by meta-analysis of randomized controlled clinical trials for isopropanolic black cohosh extract. Menopause 2011;18:366–75
- Stoll W. Phytotherapeutikum beeinflußt atrophisches Vaginalepithel: Doppelblindversuch Cimicifuga vs. Oestrogenpräparat. Therapeutikon 1987;1:23–31
- Jacobson JS, Troxel AB, Evans J, et al. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol 2001;19:2739–45
- Liske E, Hänggi W, Henneicke-von Zepelin H-H, Boblitz N, Wüstenberg P, Rahlfs VW. Physiological investigation of a unique extract of black cohosh (Cimicifugae racemosae rhizoma): a 6-month clinical study demonstrates no systemic estrogenic effect. J Womens Health Gend Based Med 2002;11:163–74
- Osmers R, Friede M, Liske E, Schnitker J, Freudenstein J, Henneicke-von Zepelin HH. Efficacy and safety of isopropanolic black cohosh extract for climacteric symptoms. Obstet Gynecol 2005;105:1074–83
- Nappi RE, Malavasi B, Brundu B, Facchinetti F. Efficacy of Cimicifuga racemosa on climacteric complaints: a randomized study versus low-dose transdermal estradiol. Gynecol Endocrinol 2005;20:30–5
- Uebelhack R, Blohmer JU, Graubaum HJ, Busch R, Gruenwald J, Wernecke KD. Black cohosh and St. John's wort for climacteric complaints: a randomized trial. Obstet Gynecol 2006;107:247–55
- Bai W, Henneicke-von Zepelin H-H, Wang S, et al. Efficacy and tolerability of a medicinal product containing an isopropanolic black cohosh extract in Chinese women with menopausal symptoms: a randomized, double blind, parallel-controlled study versus tibolone. Maturitas 2007;58:31–41
- Li Y, Cui M, Gao S. Efficacy of remifemin for control of climacteric symptoms. Prog Obstet Gynecol 2011;20:462–5
- Sun N-x, Jin Z-j, Jia X-f, Li W. Black cohosh improves vaginal atrophy in postmenopausal women. Academic Journal of Second Military Medical University 2012;32:339–41
- Huang YX, Song L, Zhang X, Lun WW, Pan C, Huang YS. [Clinical study of combined treatment of remifemin and paroxetine for perimenopausal depression]. Zhonghua Yi Xue Za Zhi 2013;93:600–2
- Xi S, Liske E, Wang S, et al. Effect of isopropanolic Cimicifuga racemosa extract on uterine fibroids in comparison with tibolone among patients of a recent randomized, double blind, parallel-controlled study in Chinese women with menopausal symptoms. Evid Based Complement Alternat Med 2014;2014:717686
- Chen J, Gao H, Li Q, et al. Efficacy and safety of remifemin on peri-menopausal symptoms induced by post-operative GnRH-a therapy for endometriosis: a randomized study versus tibolone. Med Sci Monit 2014;20:1950–7
- Jiang K, Jin Y, Huang L, et al. Black cohosh improves objective sleep in postmenopausal women with sleep disturbance. Climacteric 2015;18:559–67
- Briese V, Stammwitz U, Friede M, Henneicke-von Zepelin HH. Black cohosh with or without St. John's wort for symptom-specific climacteric treatment – results of a large-scale, controlled, observational study. Maturitas 2007;57:405–14
- Garcia-Perez MA, Pineda B, Hermenegildo C, Tarin JJ, Cano A. Isopropanolic Cimicifuga racemosa is favorable on bone markers but neutral on an osteoblastic cell line. Fertil Steril 2009;91:1347–50
- Lundström E, Hirschberg AL, Soderqvist G. Digitized assessment of mammographic breast density – effects of continuous combined hormone therapy, tibolone and black cohosh compared to placebo. Maturitas 2011;70:361–4
- Li W, Sun N-X, Chen X, Lang D-F, Jin Z-J. 早期子宫内膜癌患者术后应用黑升麻制剂治疗绝经相关症状 [Cimicifuga racemosa for treatment of menopausal symptoms in patients with early endometrial cancer after operation]. Acad J Second Mil Med Univ 2013;32:562–4
- Henneicke-von Zepelin HH, Meden H, Kostev K, Schröder-Bernhardi D, Stammwitz U, Becher H. Isopropanolic black cohosh extract and recurrence-free survival after breast cancer. Int J Clin Pharmacol Ther 2007;45:143–54
- Rebbeck TR, Troxel AB, Norman S, et al. A retrospective case-control study of the use of hormone-related supplements and association with breast cancer. Int J Cancer 2007;120:1523–8
- Obi N, Chang-Claude J, Berger J, et al. The use of herbal preparations to alleviate climacteric disorders and risk of postmenopausal breast cancer in a German case-control study. Cancer Epidemiol Biomarkers Prev 2009;18:2207–13
- Liske E. Phytokombination lindert psychovegetative Leiden. [Phytocombination alleviates psychovegatative disorders]. TW Gynäkologie 1997;10:172–5
- Nesselhut T, Liske E, ed. Pharmacological measures in postmenopausal women with an isopropanolic aqueous extract of Cimicifugae racemosae rhizoma. 10th Annual Meeting of the North American Menopause Society (NAMS); 1999: Menopause.
- Pockaj BA, Loprinzi CL, Sloan JA, et al. Pilot evaluation of black cohosh for the treatment of hot flashes in women. Cancer Invest 2004;22:515–21
- Schmidt M, Polasek W, Käufeler R. Efficacy and safety of black cohosh (Cimicifuga racemosa, Cimifemin®) in menopause discomfort: surveillance study in practical terms [Wirksamkeit und Sicherheit von Traubensilberkerze (Cimicifuga racemosa, Cimifemin®) bei Menopausebeschwerden: Therapiebeobachtung unter Praxisbedingungen]. J Für Menopause 2005;1:27–32
- Vermes G, Banhidy F, Acs N. The effects of Remifemin(R) on subjective symptoms of menopause. Adv Therapy 2005;22:148–54
- Hirschberg AL, Edlund M, Svane G, Azavedo E, Skoog L, Schoultz BV. An isopropanolic extract of black cohosh does not increase mammographic breast density or breast cell proliferation in postmenopausal women. Menopause 2007;14:89–96
- Arriaza Peso E, del Carmen Arévalo Páez M, Ángeles Grandas Alonso M, Olleros Izard T. Eficacia de Cimicifuga racemosa para el tratamiento de la clínica vasomotora y psíquica en pacientes menopáusicas. [Efficacy of Cimicifuga racemosa in the treatment of vasomotor and psychic symptoms in menopausal patients]. Progresos de Obstetricia y Ginecología 2008;51:20–7
- Reame NE, Lukacs JL, Padmanabhan V, Eyvazzadeh AD, Smith YR, Zubieta JK. Black cohosh has central opioid activity in postmenopausal women: evidence from naloxone blockade and positron emission tomography neuroimaging. Menopause 2008;15:832–40
- Molla J, Garcia-Sanchez Y, Romeu Sarri A, Perez-Lopez FR. Cimicifuga racemosa treatment and health related quality of life in post-menopausal Spanish women. Gynecol Endocrinol 2009;25:21–6
- Wu X. 莉芙敏改善妇科恶性肿瘤患者术后绝经综合征的临床研究 [Remifemin improve gynecological malignant tumor postoperative patients of menopause syndrome for the clinical research]. Dalian Medical University: Master Degree Thesis; 2011
- Rostock M, Fischer J, Mumm A, Stammwitz U, Saller R, Bartsch HH. Black cohosh (Cimicifuga racemosa) in tamoxifen-treated breast cancer patients with climacteric complaints – a prospective observational study. Gynecol Endocrinol 2011;27:844–8
- Wang C, Huang Q, Liang CL, et al. Effect of cimicifuga racemosa on menopausal syndrome caused by LHRH-a in breast cancer. J Ethnopharmacol 2019;238:111840
- Hofstatter EW, Trant AA, Stavris K, et al. The effect of black cohosh on Ki67 levels in DCIS patients. JCO 2018;36:e13541–e13541
- Chen J-y. 莉芙敏与利维爱治疗围绝经期综合征的临床疗效观察 [Comparison on the efficacy and safety of Remifemin and Livial in the treatment of perimenopausal symptoms]. Hainan Med J 2013;24:1454–7
- Zhang Q, Zhuang Z, Chen T. 莉芙敏治疗围绝经期综合征的安全性和疗效研究 [The efficacy and safety of Remifemin in the treatment of perimenopausae syndrome]. Journal of Chengdu Medical College 2015;10:229–32
- Henneicke-von Zepelin H-H, ed. High-dose isopropanolic Cimicifuga racemosa-extract for climacteric complaints in perimenopausal women. Human Reproduction, 12th World Congress; 2005; Venice
- Huang X. 黑升麻根茎提取物缓解乳腺癌患者绝经症状的临床研究 [The clinical research of Black cohosh extract in breast cancer patients with climacteric complaints]. Nanjing University of Chinese Medicine: Master Degree Thesis; 2011
- Sarri G, Pedder H, Dias S, Guo Y, Lumsden MA. Vasomotor symptoms resulting from natural menopause: a systematic review and network meta-analysis of treatment effects from the National Institute for Health and Care Excellence guideline on menopause. BJOG 2017;124:1514–23
- Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry 2006;63:375–82
- Minuzzi L, Frey BN, Soares CN. Depression during the menopausal transition: an update on epidemiology and biological treatments. FOC 2012;10:22–7
- Lehmann-Willenbrock E, Riedel HH. Klinische und endokrinologische Untersuchungen zur Therapie ovarieller Ausfallerscheinungen nach Hysterektomie unter Belassung der Adnexe. Zent bl Gynäkol 1988;110:611–18
- Ruan X, Mueck AO, Beer AM, Naser B, Pickartz S. Benefit-risk profile of black cohosh (isopropanolic Cimicifuga racemosa extract) with and without St John's wort in breast cancer patients. Climacteric 2019;22:339–47
- Hadji P. Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol Hematol 2010;73:156–66
- Fallowfield L. Acceptance of adjuvant therapy and quality of life issues. Breast 2005;14:612–16
- Assessment report on Cimicifuga racemosa (L.) Nutt., rhizoma [Internet]. 2011. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2011/05/WC500106358.pdf
- Teschke R, Schwarzenboeck A. Suspected hepatotoxicity by Cimicifugae racemosae rhizoma (black cohosh, root): critical analysis and structured causality assessment. Phytomedicine 2009;16:72–84
- Teschke R. Black cohosh and suspected hepatotoxicity: inconsistencies, confounding variables, and prospective use of a diagnostic causality algorithm. A critical review. Menopause 2010;17:426–40
- Teschke R, Bahre R, Fuchs J, Wolff A. Black cohosh hepatotoxicity: quantitative causality evaluation in nine suspected cases. Menopause 2009;16:956–65
- Teschke R, Schmidt-Taenzer W, Wolff A. Spontaneous reports of assumed herbal hepatotoxicity by black cohosh: is the liver-unspecific Naranjo scale precise enough to ascertain causality? Pharmacoepidemiol Drug Saf 2011;20:567–82
- Struck D, Tegtmeier M, Harnischfeger G. Flavones in extracts of Cimicifuga racemosa. Planta Med 1997;63:289
- Kennelly EJ, Baggett S, Nuntanakorn P, et al. Analysis of thirteen populations of black cohosh for formononetin. Phytomedicine 2002;9:461–7
- Beck V, Unterrieder E, Krenn L, Kubelka W, Jungbauer A. Comparison of hormonal activity (estrogen, androgen and progestin) of standardized plant extracts for large scale use in hormone replacement therapy. J Steroid Biochem Mol Biol 2003;84:259–68
- Nisslein T, Koetter U, Freudenstein J. In vitro binding of an isopropanolic extract of black cohosh to selected central nervous receptors. Maturitas 2006;54:S65
- Burdette JE, Liu J, Chen SN, Fabricant DS, et al. Black cohosh acts as a mixed competitive ligand and partial agonist of the serotonin receptor. J Agric Food Chem 2003;51:5661–70
- Rhyu MR, Lu J, Webster DE, Fabricant DS, Farnsworth NR, Wang ZJ. Black cohosh (Actaea racemosa, Cimicifuga racemosa) behaves as a mixed competitive ligand and partial agonist at the human mu opiate receptor. J Agric Food Chem 2006;54:9852–7
- Hui Z, Xiaoyan M, Mukun Y, et al. Effects of black cohosh and estrogen on the hypothalamic nuclei of ovariectomized rats at different temperatures. J Ethnopharmacol 2012;142:769–75
- Garcia de Arriba S, Henneicke v, Zepelin H-H, Dimpfel W, Nolte K. Cimicifuga extract modulates brain activity: electropharmacogram from various brain areas in freely moving rats (Tele-Stereo-EEG). Maturitas 2015;82:318
- Menopause: diagnosis and management. NICE guideline (NG23). Version 1.5 [Internet]. 2015. //www.nice.org.uk/guidance/ng23 https://www.nice.org.uk/guidance/ng23/evidence/full-guideline-pdf-559549261
- Bodinet C, Freudenstein J. Influence of Cimicifuga racemosa on the proliferation of estrogen receptor-positive human breast cancer cells. Breast Cancer Res Treat 2002;76:1–10
- Bodinet C, Freudenstein J. Influence of marketed herbal menopause preparations on MCF-7 cell proliferation. Menopause 2004;11:281–9
- Hostanska K, Nisslein T, Freudenstein J, Reichling J, Saller R. Cimicifuga racemosa extract inhibits proliferation of estrogen receptor-positive and negative human breast carcinoma cell lines by induction of apoptosis. Breast Cancer Res Treat 2004;84:151–60
- Hostanska K, Nisslein T, Freudenstein J, Reichling J, Saller R. Inhibitory effect of an isopropanolic extract of black cohosh on the invasiveness of MDA-MB 231 human breast cancer cells. In Vivo 2007;21:349–55
- Nesselhut T, Bodinet C, Schneider P, Freudenstein J. Use of extract of Cimicifuga racemosa. US Patent No. 6,267,994 B1 Jul. 31, 2001
- Crone M, Hallman K, Lloyd V, et al. The antiestrogenic effects of black cohosh on BRCA1 and steroid receptors in breast cancer cells. Breast Cancer (Dove Med Press) 2019;11:99–110
- Freudenstein J, Dasenbrock C, Nisslein T. Lack of promotion of estrogen-dependent mammary gland tumors in vivo by an isopropanolic Cimicifuga racemosa extract. Cancer Res 2002;62:3448–52
- Nisslein T, Freudenstein J, ed. Prepubertal treatment with black cohosh attenuates pathogenicity in the DMBA rat model of mammary carcinoma. 10th world congress on the menopause; 2002 P06-08; Berlin: Climacteric
- Nisslein T, Freudenstein J, ed. Synergistic effects of Black Cohosh and Tamoxifen in an animal model of mammary carcinoma. 6th European Congress on Menopause; 2003: Maturitas
- Einbond LS, Su T, Wu HA, et al. The growth inhibitory effect of actein on human breast cancer cells is associated with activation of stress response pathways. Int J Cancer 2007;121:2073–83
- Yue GG, Xie S, Lee JK, et al. New potential beneficial effects of actein, a triterpene glycoside isolated from Cimicifuga species, in breast cancer treatment. Sci Rep 2016;6:35263
- Ji L, Zhong B, Jiang X, et al. Actein induces autophagy and apoptosis in human bladder cancer by potentiating ROS/JNK and inhibiting AKT pathways. Oncotarget 2017;8:112498–515
- Paplomata E, ÓRegan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther Adv Med Oncol 2014;6:154–66
- Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol 2014;4:64
- Moser C, Vickers SP, Brammer R, Cheetham SC, Drewe J. Antidiabetic effects of the Cimicifuga racemosa extract Ze 450 in vitro and in vivo in ob/ob mice. Phytomedicine 2014;21:1382–9
- Li B, Takeda H, Tsuiji K, et al. The antidiabetic drug metformin inhibits uterine leiomyoma cell proliferation via an AMP-activated protein kinase signaling pathway. Gynecol Endocrinol 2013;29:97–0
- Tadakawa M, Takeda T, Li B, Tsuiji K, Yaegashi N. The anti-diabetic drug metformin inhibits vascular endothelial growth factor expression via the mammalian target of rapamycin complex 1/hypoxia-inducible factor-1α signaling pathway in ELT-3 cells. Mol Cell Endocrinol 2015;399:1–8
- Ciarmela P, Islam MS, Reis FM, et al. Growth factors and myometrium: biological effects in uterine fibroid and possible clinical implications. Hum Reprod Update 2011;17:772–90
- Viereck V, Grundker C, Friess SC, et al. Isopropanolic extract of black cohosh stimulates osteoprotegerin production by human osteoblasts. J Bone Miner Res 2005;20:2036–43
- Stute P, Pickartz S. Zusatznutzen eines isopropanolischen Cimicifuga-racemosa-Extrakts (iCR): positive Beeinflussung von Knochenstoffwechsel und -qualität. Z Phytother 2015;36:18–22
- Nisslein T, Freudenstein J. Effects of an isopropanolic extract of Cimicifuga racemosa on urinary crosslinks and other parameters of bone quality in an ovariectomized rat model of osteoporosis. J Bone Miner Metab 2003;21:370–6
- Cui G, Leng H, Wang K, et al. Effects of remifemin treatment on bone integrity and remodeling in rats with ovariectomy-induced osteoporosis. PLoS One 2013;8:e82815
- Qiu SX, Dan C, Ding L-S, et al. A triterpene glycoside from black cohosh that inhibits osteoclastogenesis by modulating RANKL and TNFalpha signaling pathways. Chem Biol 2007;14:860–9
- Biglia N, Cagnacci A, Gambacciani M, Lello S, Maffei S, Nappi RE. Vasomotor symptoms in menopause: a biomarker of cardiovascular disease risk and other chronic diseases? Climacteric 2017;20:306–12
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 2010;49:1603–16
- Islam MS, Akhtar MM, Ciavattini A, et al. Use of dietary phytochemicals to target inflammation, fibrosis, proliferation, and angiogenesis in uterine tissues: promising options for prevention and treatment of uterine fibroids? Mol Nutr Food Res 2014;58:1667–84
- Gordon JL, Rubinow DR, Thurston RC, Paulson J, Schmidt PJ, Girdler SS. Cardiovascular, hemodynamic, neuroendocrine, and inflammatory markers in women with and without vasomotor symptoms. Menopause 2016;23:1189–98
- Da Y, Niu K, Wang K, et al. A comparison of the effects of estrogen and Cimicifuga racemosa on the lacrimal gland and submandibular gland in ovariectomized rats. PLoS One 2015;10:e0121470
- Yang CL, Or TC, Ho MH, Lau AS. Scientific basis of botanical medicine as alternative remedies for rheumatoid arthritis. Clin Rev Allergy Immunol 2013;44:284–300