Abstract
Objective
Oral but not transdermal menopausal hormone therapy (MHT) increases the risk of venous thromboembolism. There is no evidence regarding the risk of the serious complication pulmonary embolism (PE). The aim was to investigate the risk of PE in women using MHT depending on administration route, type of progestin and treatment duration.
Method
The population-based case-control study covered 1,771,253 women aged 40–69 years, during 2006–2015. Diagnoses of PE (n = 13,974) and drug dispensations were received from national validated registers.
Results
Current MHT users had a higher risk of PE than non-users (odds ratio [OR] 1.15, 95% confidence interval [CI] 1.05–1.26). First ever users had the highest risk (OR 2.07, 95% CI 1.23–3.50). Transdermal administration was not associated with increased risk of PE. The OR was slightly but non-significantly higher with estrogen combined with medroxyprogesterone acetate than with norethisterone acetate.
Discussion
The risk of PE was significantly increased in users of oral but not transdermal MHT, with the highest risk in first ever users of oral estrogen combined with medroxyprogesterone acetate. The risk was considerably lower in women with recurrent treatment, probably because of the healthy user effect.
Conclusion
PE was most common close to initiation of oral treatment. Transdermal MHT did not increase the risk of PE.
摘要
目的:口服而非经皮的绝经激素治疗(MHT)会增加静脉血栓栓塞的风险。目前还没有证据表明严重并发症肺栓塞(PE)的风险。目的是研究使用MHT的妇女发生PE的风险, 这取决于给药途径、孕激素的类型和治疗时间。
方法:这项基于人群的病例对照研究涵盖了2006-2015年期间1,771,253名40-69岁的妇女。PE的诊断(n= 13,974)和药物配置是从国家有效的登记册中得到的。
结果:目前应用MHT的人比不应用的人有更高的PE风险(几率比[OR]1.15, 95%置信区间[CI]1.05-1.26)。首次应用者的风险最高(OR 2.07, 95% CI 1.23-3.50)。经皮给药与PE风险的增加没有关系。雌激素联合醋酸甲羟孕酮的OR值略高于醋酸炔诺酮, 但并不明显。
讨论:口服而非经皮MHT的使用者发生PE的风险显著增加, 首次应用口服雌激素联合醋酸甲羟孕酮的使用者风险最高。在反复治疗的妇女中, 风险大大降低, 可能是因为健康用户效应。
结论:PE在开始口服治疗时最常见。经皮MHT并不增加PE的风险。
Introduction
Approximately 60–80% of all women experience vasomotor symptoms at some point during the menopausal transition [Citation1–4] with a mean duration of around 5 years [Citation3,Citation5,Citation6]. The symptoms are often most pronounced in the years around menopause [Citation1,Citation2,Citation4,Citation7]. Systemic menopausal hormone therapy (MHT) is considered the most effective medical treatment against vasomotor symptoms, with a reported symptom reduction of 75–95% [Citation3,Citation6,Citation8]. MHT contains either estrogen in monotherapy (hysterectomized women) or estrogen in combination with a progestin to avoid endometrial hyperstimulation and hyperplasia. Systemic administration can be either oral or transdermal. There are also low-dose preparations for the treatment of local urogenital symptoms, which are considered to have very limited systemic effects and do not have to be combined with a progestin [Citation9].
MHT is a known risk factor for venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE). Age per se is also a strong risk factor for VTE [Citation10], with a more than doubled incidence in Sweden for women aged 65–69 years compared to those aged 40–44 years [Citation10]. Other risk factors are pregnancy, surgery, immobilization, obesity, cancer and a genetic predisposition/family history [Citation11].
Several studies have found two to three times increased risk for VTE in current MHT users compared with non-users. The risk increase has been reported to be most pronounced during the first 2 years of drug use [Citation12–17] and to disappear after treatment is finished [Citation15]. Lately, it has become more evident that the increased VTE risk is mainly related to the use of oral but not transdermal MHT [Citation14,Citation15,Citation18–22]. Unlike oral MHT, the transdermal route avoids first-pass metabolism in the liver, which affects the production and activation of clotting factors [Citation23].
MHT containing estrogen in combination with a progestin seems to increase the risk of VTE more than estrogen in monotherapy [Citation12,Citation16,Citation24,Citation25]. Some studies have also found differences in risk estimates between MHT containing different progestins, most pronounced for medroxyprogesterone acetate (MPA) [Citation15,Citation18,Citation20]. Micronized progesterone in combination with estrogen has been suggested to be the combined oral MHT associated with the lowest risk of VTE [Citation14,Citation18,Citation22,Citation26]. This regimen is currently not available in Sweden as a registered MHT. MHT with the progestin dydrogesterone, a progestin very similar to progesterone, has not been related to an increased VTE risk [Citation20,Citation27,Citation28]. This preparation, however, has only been available in Sweden since 2019, and there are as yet no Swedish data on VTE risk with such preparations.
According to Swedish and international recommendations, transdermal treatment is probably a safer alternative for women with risk factors for VTE [Citation9,Citation29,Citation30], even though the position statement of The North American Menopause Society requests more and urgent research on the subject [Citation31]. Thus, and despite the well-established knowledge that MHT increases the risk of VTE, there is a need for more data on the role of different regimes and progestins combined with estrogen, especially for the risk of PE which is the most serious venous thromboembolic event.
However, only a few studies have analyzed the risk of DVT and PE separately depending on the route of administration of MHT. A systematic review [Citation32] from 2015 found only one study analyzing the risk of PE. This study, considered to be of low quality, reported no significant increase in the risk of PE with oral compared to transdermal estrogen therapy (relative risk, 2.0; 95% confidence interval [CI], 0.81–4.95) [Citation33].
In Sweden, data on inpatient and outpatient specialist care are reported to the National Patient Register, with complete coverage of the inpatient diagnoses since 1987. An external review and validation from 2011 found a positive predictive value of 85–95% for diagnoses in the inpatient register [Citation34]. Diagnoses from the specialized outpatient care were missing in 80% of registered contacts in 2007, and data from primary care and several private caregivers are not included [Citation35]. Regarding VTE, PE is most likely to be diagnosed and treated in inpatient care, while DVT might be diagnosed in outpatient care. Consequently, register-retrieved diagnoses of PE in Sweden should be considered more reliable than diagnoses of DVT.
Aim
The overall aim of this study was to investigate the risk of PE in women aged 40–69 years using MHT in Sweden during 2006–2015.
The primary aim was to assess the association between the use of MHT and PE depending on the route of administration and the duration of drug use.
The secondary aim was to assess the association between the use of MHT and PE, depending on the type of progestin used in combination with estrogen.
Methods
Study design
The register-based case–control study is based on the complete female population of Sweden aged 40–69 years at some point during the study period.
Study objects
The population was defined as all women aged 40–69 years registered as a resident in Sweden at some point during the period from 1 July 2005 to 31 December 2015. They were identified using the Total Population Register, held by Statistics Sweden [Citation36], and included 1,771,253 individuals. Age at index date – that is, the date when PE was diagnosed – was calculated as the year at inclusion minus the year of birth.
Women with a prior diagnosis of VTE are less likely to be prescribed MHT and were therefore excluded. The definition of VTE with International Classification of Diseases 10th Revision (ICD-10) codes is available as Supplementary material (Supplementary Table 1).
Table 1. ATC codes of available systemic menopausal hormone therapy (MHT) in Sweden 2000–2015.
Collection of data
Diagnoses of PE were identified using the National Patient Register [Citation34], held by the National Board of Health and Welfare.
The diagnoses in the register are coded using the ICD [Citation37]. The occurrence of PE was defined as the registration of ICD-10 codes I26.0 (PE with mention of acute cor pulmonale) or I26.9 (PE without mention of acute cor pulmonale) [Citation38] in the National Patient Register or the Swedish Cause of Death Register [Citation39]. The latter was also used to censor participants at death.
Data from the National Board of Health and Welfare were sent to Statistics Sweden for record linkage. The Personal Identification Number was pseudonymized by a serial number before access was granted to the researchers at Linköping University.
Definition of drug use
Data on dispensed drugs were extracted from the Swedish Prescribed Drug Register. The register covers all prescribed drugs dispensed by Swedish pharmacies, irrespective of reimbursement status, including individual-level data from 1 July 2005 [Citation40].
Systemic MHT was defined using the Anatomical Therapeutic Chemical (ATC) codes (2016) presented in . MHT was further subclassified as oral or transdermal, and by type of progestin (MPA or noretisterone acetate (NETA)). Fixed combinations of estrogen in combination with progestogen are, with one exception, only available as oral treatment in Sweden. During the study period, only one fixed preparation of transdermal estrogen + NETA was available and it constitutes a minor part of the total combined MHT.
For both cases and controls, the index date was defined as the date of diagnosis of PE for each case. The duration of treatment was calculated retrospectively from the index date. The type of MHT was defined by the preparation dispensed closest to the index date. Twelve months of dispensation history were needed to categorize MHT users as current, new or first ever users. Since dispensation data on an individual level in the Swedish Prescribed Drug Register were only available from 1 July 2005, the index date could not be included before the 1 July 2006. Ongoing treatment was defined as at least two dispensations per year consecutively ().
Figure 1. Definition of drug use and duration of menopausal hormone therapy (MHT).
Note: X, MHT dispensation. Current MHT use was defined as at least one registered dispensation from the pharmacy within 4 months prior to the index date (diagnosis of pulmonary embolism [PE]), independently of previous dispensations or not (individuals 3–8). Women with a dispensation within 0–4 months, but not 5–12 months, before the index date were considered new users (individual 4). If they did not have any dispensation even further back in time, they were considered first ever users (individual 3). Ongoing treatment was defined as at least two dispensations per year consecutively. Women without any dispensation during the study period were considered non-users (individual 2). Women with dispensations that did not meet the criteria for continuous treatment were considered previous users and excluded from the analysis (individual 1).
![Figure 1. Definition of drug use and duration of menopausal hormone therapy (MHT).Note: X, MHT dispensation. Current MHT use was defined as at least one registered dispensation from the pharmacy within 4 months prior to the index date (diagnosis of pulmonary embolism [PE]), independently of previous dispensations or not (individuals 3–8). Women with a dispensation within 0–4 months, but not 5–12 months, before the index date were considered new users (individual 4). If they did not have any dispensation even further back in time, they were considered first ever users (individual 3). Ongoing treatment was defined as at least two dispensations per year consecutively. Women without any dispensation during the study period were considered non-users (individual 2). Women with dispensations that did not meet the criteria for continuous treatment were considered previous users and excluded from the analysis (individual 1).](/cms/asset/a719a1aa-c997-457b-99c4-9599e7c66b1b/icmt_a_2127352_f0001_c.jpg)
For example, a treatment duration of 2 years was defined as individual 6 in fulfilling all three of the following criteria:
At least two dispensations within the first year prior to the index date (defined as one dispensation within 0–4 months, and in addition one dispensation during the 5–12 months preceding the index date).
At least two dispensations 13–24 months prior to the index date.
Less than two dispensations in the third year prior to the index date.
Statistical analysis
Since the cases of PE were matched to controls of the same age and who were included in the same year, we used conditional logistic regression for the analyses. Cases of PE were matched by age and year of inclusion. The number of controls varied between four and 19, with a mean of 15.3. p < 0.05 was considered statistically significant throughout the study.
Stata/MP17.1 (StataCorp LLC, College Station, TX, USA) was used for statistical analyses. Figures were created using Microsoft Excel version 15.4.
Ethical consideration
The Regional Ethical Review Board in Linköping Sweden approved this register study (D-nr 2012/386-31 and 2016/130-32). Data were extracted from national mandatory population health registers by the National Board of Health and Welfare and Statistics Sweden. The data management is regulated by Swedish law [Citation41], and thus no informed consent was needed [Citation42].
Results
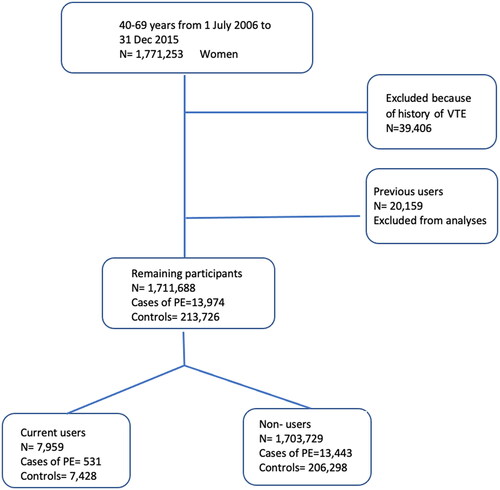
Among the 1,771,253 women aged 40–69 years identified in the Total Population Register, 59,565 were excluded because of previous VTE or use of MHT that could not be classified as current due to our definition. After exclusion, 13,974 women remained with a diagnosis of PE during the period from 1 July 2006 to 31 December 2015 (see for a flowchart).
Figure 2. Flowchart showing the number of included and excluded cases of pulmonary embolism (PE) and controls. VTE, venous thromboembolism.
Current users of MHT had a significantly increased risk of PE compared with non-users (odds ratio [OR] 1.15, 95% CI 1.05–1.26). Within this group, the women who were considered first ever users had the highest risk of being diagnosed with PE (OR 2.07, 95% CI 1.23–3.50). Women considered as new users according to the more liberal definition with no dispensation 5–12 months () prior to the index date had a non-significant increased risk ().
Table 2. Risk of pulmonary embolism (PE) in current, new and first ever users of menopausal hormone therapy (MHT), subclassified by route of administration and type of progestin used in combination with estrogen.
When MHT was stratified for oral and transdermal administration, the risk of PE was significantly increased in current, new and first ever users of oral MHT. Users of oral MHT had slightly higher OR than MHT in total, whereas transdermal use was not associated with an increased risk of PE ( and ).
Table 3. Risk of pulmonary embolism (PE) depending on the duration of menopausal hormone therapy (MHT), subclassified by route of administration.
For longer treatment duration with MHT in total, no significantly increased risk was observed for each year of use (). Women using oral MHT had an increased risk of PE for each separate year, but the risk was statistically significant only for the first, fourth and eighth years ().
The OR for PE was higher in current users of MHT with estrogen combined with MPA (OR 1.60, 95% CI 1.31–1.95) than for users of estrogen in combination with NETA (OR 1.39, 95% CI 1.20–1.61), although the 95% CI overlapped. In new and first ever users, the number of users in each progestin group was too low to reach statistical significance (except for new users of estrogen + MPA), but a similar trend was seen.
Discussion
The essential finding of this study was that first ever users of MHT had an increased risk of PE within 4 months after starting therapy, compared with non-users. However, we found a difference in the risk of PE between true first ever users and women considered new users based on a run-in period (of 8 months). This can probably be explained by a healthy user effect; that is, that women who discontinue treatment due to reasons other than serious side effects are more likely to be recurrent users than women who discontinue because of, for example, PE. Women on MHT who, for some reason other than VTE, discontinue their treatment are less likely to experience PE if they restart after a few months or years with a similar baseline risk since they have already passed the critical first months of use once.
In line with previous findings regarding VTE, the risk of PE was increased only in women using oral treatment. We could not identify any increased risk of PE in users of transdermal MHT. To the best of our knowledge, this has not previously been shown for PE but is in accordance with previous studies of MHT and VTE overall [Citation18,Citation33]. When studying the duration of treatment in years, there was a clear trend toward a gradual risk increase over the first 4 years of oral MHT use. Thereafter, the numbers of cases of PE and users of MHT were probably too low to reach statistical power, despite our comprehensive data.
Since almost all previous studies on MHT and VTE were conducted with DVT as the primary outcome, our results contribute relevant knowledge about the risk of PE, which is the most severe form of VTE. Our decision to focus on PE, and not VTE in general, was based on the lack of previous studies analyzing PE and DVT separately, but also on the assumption that PE is most likely to be diagnosed in inpatient care. In contrast, DVT is often diagnosed in outpatient care with less reliable register data [Citation34]. Still, we excluded women with a history of DVT since they have an increased risk of recurrent VTE and are less likely to be prescribed MHT.
The large discrepancy between the risk of PE in first ever users and current users of oral MHT highlights that PE is an adverse event that usually occurs close to initiation of treatment.
We found a higher risk of PE in women using estrogen in combination with MPA compared to estrogen in combination with NETA. This finding is in line with several studies on VTE [Citation15,Citation20], including a large meta-analysis from 2018 [Citation18], but is in contradiction to other studies that have reported a lower risk with MPA [Citation22,Citation26]. Thus, the risk of VTE related to the type of progestin used in combination with estrogen has been insufficiently studied. This is especially true for PE, which was the focus of our study.
The main strength of this large population-based study is the coverage and reliability of the registers and their data [Citation34], which makes the results highly generalizable. We have studied all cases with a diagnosis code of PE in women in Sweden over a decade for the age groups where MHT is mainly considered. The comprehensive Swedish health-care system, which has almost complete registration of inpatient diagnoses, offers a unique opportunity to study rare outcomes, such as PE. Also, all relevant drugs are included in the drug benefit system, which further minimizes the risk of selection bias. The fact that we analyzed repeated actual dispensations of MHT, and not only issued prescriptions, is also considered a major strength of this study since it makes it more probable that individuals actually have used the studied drug.
Still, PE is a rare condition. Even though we had data for a population of more than 1.7 million women and more than 200,000 women were included in each analysis, the number of women on MHT diagnosed with PE was limited. Previous studies have suggested a dose-dependent effect of oral but not transdermal estradiol on coagulation and the subsequent thromboembolic effect [Citation20]. It would have been interesting to analyze the PE risk in relation to the dose of estrogen, but we did not have information about dispensed doses. Still, even if we had the data, it would probably not be possible to make such analyses due to lack of power. The same applies to comparisons between other progestins, tibolone and sequential versus continuous treatment. It would have been desirable to further analyze the treatment duration and risk of PE with different progestins, but the results would not have been reliable because of limited data and, hence, low power in multiple analyses. Therefore, we chose to limit the analyses on different progestins (MPA and NETA) to current, new and first ever users. More data will accumulate and become available in the future, and further analyses can be made in international studies, perhaps in the Nordic countries with similar demographics and health-care systems. New products such as micronized progesterone and dydrogesterone have recently been introduced in Sweden. MHT with these progestins might have a different risk of VTE [Citation20,Citation27,Citation28].
We initially planned for 20 controls per PE case, but due to limited availability this was not possible. Another limitation is the follow-up time for participants included early in the study period since the individual-level data were only included in the Swedish Prescribed Drug Register from 1 July 2005. The limitations of follow-up in the early years studied might imply that a person may have used MHT further back in time, but we lack information about it. This might result in a situation when a woman, who is in fact a recurrent user of MHT, will be misclassified as a first ever user. The same might be true with women who immigrated to Sweden during the study period. However, this would not reduce the relevance of our results since a correction of the misclassification would instead have made the difference even more pronounced between the groups studied. Another weakness is that some women might historically have used more than one type of MHT; that is, both oral and transdermal preparations or different progestins. This was not corrected for in this study.
A general limitation with register-based studies is the lack of some information. Co-morbidity, menopausal status, hysterectomies, oophorectomies and smoking habits are some examples of data on the individual level that would have improved the analyses. Our findings can only be generalized for the age group studied, and not for women using MHT for the indication of premature ovarian insufficiency.
Conclusion
The risk of PE was highest in first ever users and was considerably lower in women who might have used MHT previously. Estrogen in combination with both MPA and NETA was associated with an increased risk of PE. The risk was significantly increased in the first 4 years in users of oral MHT, but not in transdermal users.
Potential conflict of interest
Nil.
Supplemental Material
Download MS Word (25.4 KB)Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Thurston RC, Joffe H. Vasomotor symptoms and menopause: findings from the study of women’s health across the nation. Obstet Gynecol Clin North Am. 2011;38(3):489–501.
- Ziv-Gal A, Flaws JA. Factors that may influence the experience of hot flushes by healthy Middle-aged women. J Womens Health. 2010;19(10):1905–1914.
- Andrikoula M, Prelevic G. Menopausal hot flushes revisited. Climacteric. 2009;12(1):3–15.
- Freeman EW, Sherif K. Prevalence of hot flushes and night sweats around the world: a systematic review. Climacteric. 2007;10(3):197–214.
- Col NF, Guthrie JR, Politi M, et al. Duration of vasomotor symptoms in middle-aged women: a longitudinal study. Menopause. 2009;16(3):453–457.
- Stearns V, Ullmer L, Lopez JF, et al. Hot flushes. Lancet. 2002;360(9348):1851–1861.
- Crawford SL. The roles of biologic and nonbiologic factors in cultural differences in vasomotor symptoms measured by surveys. Menopause. 2007;14(4):725–733.
- MacLennan AHBJ, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes (review). MacLennan AH, BroadbentJL, Lester S, Moore V. 2009. UK: John Wiley & Sons, Ltd.
- Neves ECM, Birkhauser M, Samsioe G, et al. EMAS position statement: the ten point guide to the integral management of menopausal health. Maturitas. 2015;81(1):88–92.
- Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):3–14.
- Bergendal A, Bremme K, Hedenmalm K, et al. Risk factors for venous thromboembolism in pre-and postmenopausal women. Thromb Res. 2012;130(4):596–601.
- Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. Jama. 2004;292(13):1573–1580. Oct 6
- Roach RE, Lijfering WM, Helmerhorst FM, et al. The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy. J Thromb Haemost. 2013;11(1):124–131.
- Canonico M. Hormone therapy and risk of venous thromboembolism among postmenopausal women. Maturitas. 2015;82(3):304–307.
- Sweetland S, Beral V, Balkwill A, et al. Venous thromboembolism risk in relation to use of different types of postmenopausal hormone therapy in a large prospective study. J Thromb Haemost. 2012;10(11):2277–2286.
- Curb JD, Prentice RL, Bray PF, et al. Venous thrombosis and conjugated equine estrogen in women without a uterus. Arch Intern Med. 2006;166(7):772–780.
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the women’s health initiative randomized controlled trial. Jama. 2002;288(3):321–333.
- Scarabin PY. Progestogens and venous thromboembolism in menopausal women: an updated oral versus transdermal estrogen Meta-analysis. Climacteric. 2018;21(4):341–345.
- Canonico M, Plu-Bureau G, Lowe GD, et al. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and Meta-analysis. BMJ. 2008;336(7655):1227–1231.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
- Canonico M. Hormone therapy and hemostasis among postmenopausal women: a review. Menopause. 2014;21(7):753–762.
- Canonico M, Oger E, Plu-Bureau G, et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115(7):840–845.
- Fritz MA. Clinical gynecologic endocrinology and infertility. Philadelphia, USA: Lippincott Williams & Wilkins, a Wolters Kluwer business; 2011. (8, editor.).
- Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the women’s health initiative randomized controlled trial. Jama. 2004;291(14):1701–1712.
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280(7):605–613.
- Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30(2):340–345.
- Stevenson JC, Panay N, Pexman-Fieth C. Pexman-Fieth C. Oral estradiol and dydrogesterone combination therapy in postmenopausal women: review of efficacy and safety. Maturitas. 2013;76(1):10–21.
- Schneider C, Jick SS, Meier CR. Risk of cardiovascular outcomes in users of estradiol/dydrogesterone or other HRT preparations. Climacteric. 2009;12(5):445–453.
- Swedish Society of Obstetrics and Gynecology. SFOG-Råd för menopausal hormonbehandling 2019, uppdaterad version 2021; [cited 2019 Dec 09]. Available from: https://www.sfog.se/media/337273/mht-sfog-raad-210121.pdf.
- Baber RJ, Panay N, Fenton A. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric. 2016;19(2):109–150.
- The 2017 hormone therapy position statement of The North American menopause society. Menopause. 2017;24(7):728–753.
- Mohammed K, Abu Dabrh AM, Benkhadra K, et al. Oral vs transdermal estrogen therapy and vascular events: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(11):4012–4020.
- Laliberté F, Dea K, Duh MS, et al. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2018;25(11):1297–1305.
- Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450.
- National board of Health and Welfare. Kvalitet och inneha°ll i patientregistret Utskrivningar fra°n slutenva°rden 1964–2007 och besök i specialiserad öppenva°rd (exklusive primärva°rdsbesök) 1997–2007. 2009.
- Statistics Sweden. DESCRIPTION OF THE REGISTER Total Population Register. 2016.
- World Health Organization. International Classification of Diseases (ICD) Information Sheet; [cited 2020 May 18]. Available from: https://www.who.int/classifications/icd/factsheet/en/.
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10)-WHO Version for; 2019; [cited 2020 May 18]. Available from: https://icd.who.int/browse10/2019/en#/I26.
- Brooke HL, Talback M, Hornblad J, et al. The swedish cause of death register. Eur J Epidemiol. 2017;32(9):765–773.
- Wettermark B, Hammar N, Fored CM, et al. The new swedish prescribed drug register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–735.
- The Riksdag. The Act concerning the Ethical Review of Research Involving Humans (SFS 2003:460) Stockholm: The Riksdag; [cited 2017 Oct 11]. Available from: https://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/lag-2003460-om-etikprovning-av-forskning-som_sfs-2003-460.
- Sveriges Riksdag. Offentlighets- och sekretesslag (2009:400) 2009; [cited 2019 Jun 3]. Available from: https://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/offentlighets–och-sekretesslag-2009400_sfs-2009-400.

