Abstract
Objective
This study aimed to evaluate the efficacy and safety of oral ultra-low-dose continuous combination of 17β-estradiol (17β-E2) and norethisterone acetate (NETA) in postmenopausal Brazilian women.
Methods
Postmenopausal women (age 45–60 years) with amenorrhea >12 months and intact uterus, with moderate to severe vasomotor symptoms, were included. The vasomotor symptoms and endometrial bleeding were evaluated by a daily diary for 24 weeks, and the women were assessed at baseline and endpoint.
Results
A total of 118 women were included. The group treated with 0.5 mg 17β-E2/0.1 mg NETA (n = 58) showed a percentage reduction of 77.1% in the frequency of vasomotor symptoms versus 49.9% in the placebo group (n = 60) (p = 0.0001). The severity score showed a reduction in the treatment group when compared to the placebo (p < 0.0001). The adverse events were comparable between the groups; however, in the 0.5 mg 17β-E2/0.1 mg NETA group there were more complaints of vaginal bleeding; despite that, in most cycles in both treatment groups, more than 80% of women experienced amenorrhea.
Conclusions
The combination of 0.5 mg 17β-E2/0.1 mg NETA in a continuous combination regimen was shown to be effective in reducing the frequency and severity of vasomotor symptoms in Brazilian postmenopausal women.
摘要
目的:本研究旨在评估巴西绝经后女性口服超低剂量连续联合17β-雌二醇(17β-E2)和醋酸炔诺酮(NETA)的疗效和安全性。
方法:绝经后女性(年龄45-60岁), 闭经>12个月, 子宫完整, 伴有中重度血管舒缩症状。血管舒缩症状和子宫内膜出血通过每日日记进行评估, 持续24周, 并在基线和终点对女性进行评估。
结果:共纳入118名女性。接受0.5 mg 17β-E2/0.1 mg NETA (n = 8)治疗组血管舒缩症状频率百分比减少77.1%, 而安慰剂组为49.9% (n = 60)(p = 0.0001)。与安慰剂组相比, 治疗组的严重程度评分有所降低(p < 0.0001)。两组间不良事件具有可比性;然而, 在0.5 mg 17β-E2/0.1 mg NETA组中, 阴道流血的主诉较多;尽管如此, 在两个组的大多数周期中, 超过80%的女性有闭经。
结论:在连续联合治疗方案中, 0.5 mg 17β-E2/0.1 mg NETA联合治疗可有效降低巴西绝经后女性血管舒缩症状的频率和严重程度。
Introduction
Despite several controversies over the last decade, menopause hormone therapy (HT) is considered the most effective treatment for symptoms due to ovarian failure, and the benefits outweigh the risks for most symptomatic women aged younger than 60 years or within 10 years of postmenopause [Citation1–4]. Vasomotor symptoms are the most common symptoms and affect most postmenopausal women and, although their severity, frequency and duration vary widely, they may affect the quality of life when they are moderate to severe [Citation5]. The efficacy of HT in the relief of vasomotor symptoms is well established, being considered the most effective treatment in perimenopausal and postmenopausal women [Citation2,Citation4]. A review study by the Cochrane Library assessing the efficacy of HT in the treatment of hot flushes included 24 clinical trials and showed a reduction of 75% in the frequency and 87% in the severity of symptoms, when compared to a placebo, regardless of the addition of progestin. The reduction of symptomatology with placebo was on average 30% [Citation6]. A meta-analysis assessed the effect of estrogen therapy when compared to a placebo and showed a reduction of −16.8 in the weekly number of hot flushes using oral 17β-estradiol (17β-E2) [Citation7]. Most data about HT and vasomotor symptoms are based on conventional estrogen doses; however, therapies with low doses are effective in symptom relief and are associated with a lower occurrence of vaginal bleeding and mastalgia [Citation8,Citation9].
The safety and tolerability of therapeutic agents should be considered against their clinical efficacy [Citation9]. The use of the lowest therapeutic dose remains an essential principle in clinical practice. As a result, the current guidelines recommend the use of the lowest effective dose of HT [Citation1–4]. The available evidence suggests that lower doses of HT may be well tolerated and have fewer adverse effects than standard doses. The use of a lower estrogen dose can also reduce the progestin dose required for endometrial protection. For this study, we defined the doses following the Italian Consensus Statement inspired by the Global Consensus on Menopausal Hormone Therapy in 2013 and 2016 by leading global menopause societies: an oral low dose of 1 mg/day 17β-E2 and an oral ultra-low dose of 0.5 mg/day 17β-E2 [Citation10]. The recommendation is to individualize HT by using the lowest dose aimed at a reduction of side effects without affecting the relief of vasomotor symptoms [Citation3]. A study investigating the efficacy of viable oral doses of 0.25–2 mg/day 17β-E2 showed that at least 0.5 mg of estradiol/day is required to significantly reduce vasomotor symptoms [Citation11]. Thus, the ultra-low dose of HT may be enough to reach this goal in perhaps one out of two women, while others require a higher initial hormone dose [Citation12,Citation13].
HT in combination with an ultra-low dose of 0.5 mg 17β-E2 and 0.1 mg norethisterone acetate (NETA) as a first-line oral option has shown to be effective, with high tolerability and safety [Citation12]. A study assessing 577 postmenopausal women has shown a significant reduction of moderate to severe vasomotor symptoms with an ultra-low dose of 0.5 mg 17β-E2/0.1 mg NETA or 0.5 mg 17β-E2/0.25 mg NETA from 3 weeks of treatment when compared to placebo [Citation14]. The rate of amenorrhea was approximately 90% after 6 months of treatment, and there were no significant changes in the mean endometrial thickness [Citation15]. The bleeding pattern with an ultra-low dose of 0.5 mg 17β-E2/0.1 mg NETA was assessed in a prospective study involving 169 postmenopausal women with a 52-week follow-up. The cumulative rates of amenorrhea were 67% in the first 3 months and 84% in the last months of follow-up. This low occurrence of bleeding-related adverse events, together with the beneficial effects on vasomotor symptoms, may promote treatment adherence [Citation16].
For many women, the ultra-low dose may be sufficient to decrease vasomotor symptoms [Citation12]. Thus, HT in combination with an ultra-low dose increases the possibility to individualize treatment for postmenopausal women. In a review study, Stute et al. consider that, aimed at reducing hormone exposure, the prescription of an ultra-low dose of HT could be considered 2–3 years after menopause [Citation13]. Also, the change from low dose to ultra-low dose may be an alternative to reduce the hormone dose without compromising the relief of vasomotor symptoms [Citation13]. Based on this, the main purpose of this study was to evaluate the efficacy and safety of oral HT in combination with an ultra-low dose of 0.5 mg 17β-E2/0.1 mg NETA in Brazilian postmenopausal women. Thus, the study had as its primary objective the evaluation of the improvement of vasomotor symptoms with the use of ultra-low-dose HT in postmenopausal Brazilian women, in addition to aspects related to safety, through the evaluation of clinical and laboratory parameters.
Methods
Study design and sample selection
A multicenter, double-blind, placebo-controlled, randomized clinical trial was conducted to evaluate the effect of the combination of 0.5 mg 17β-E2/0.1 mg NETA. A total of 118 women were treated in seven Brazilian sites.
Healthy women, aged between 45 and 60 years, non-hysterectomized and who experienced at least seven moderate or severe hot flushes per day or 50 per week during the screening period were enrolled. The menopausal status was defined by amenorrhea of more than 12 months and serum follicle stimulating hormone values ≥30 mIU/ml and E2 ≤ 30 pg/ml. The exclusion criteria included a history of coronary and cerebrovascular disease; venous thromboembolic disease; clinical severe depression episodes, thyroid dysfunction, hyperprolactinemia, endometrial hyperplasia or endometrial cancer; vaginal bleeding of unknown cause or endometrial thickening; breast cancer or precursor lesion for breast cancer; decompensated liver disease or gallbladder disease; systemic erythematosus lupus; estrogen-related migraine; uncontrolled systolic blood pressure (>150 mmHg or diastolic blood pressure >90 mmHg); body mass index <19 kg/m2 or >35 kg/m2; smoking >20 cigarettes; alcohol or drug addiction; and women treated with oral or non-oral HT within the last 3 months or with drugs with known effects on vasomotor symptoms, such as serotonin-noradrenaline reuptake inhibitors, clonidine, gabapentin, tibolone, methyldopa and phytoestrogens within the last 30 days. The Project was approved by the Research Ethics Committee of all participating sites and was conducted following the ethical standards outlined in the Helsinki Declaration (1983).
The study was registered and approved by the International Standard Randomized Controlled Trial Number (ISRCTN) under registration number 76005731.
Randomization and intervention protocol
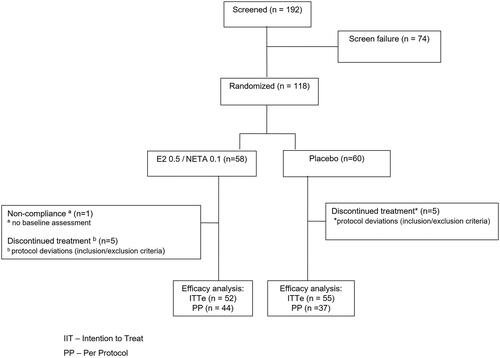
After the initial screening, women were randomly randomized at a 1:1 ratio to one of the two intervention groups: 0.5 mg 17β-E2/0.1 mg NETA, the treatment group (n = 58); or placebo (n = 60) (). The treatment consisted of tablets taken by oral route once daily for 24 weeks. For the assessment of treatment adherence, participants were instructed to bring the used or unused packages at each return. The minimum acceptable treatment adherence was 80% and the follow-up period was 24 weeks with clinical assessments at baseline and after 12 and 24 weeks.
Evaluation of effectiveness and adherence
The primary clinical efficacy endpoint was the average change in frequency and severity of vasomotor symptoms recorded in the participant’s diary between baseline and after 24-week treatment when compared to placebo. The participants had a 3-week screening period, followed by a 24-week treatment period. Throughout this period, the participants were instructed to fill out a daily diary with information regarding the number and severity of vasomotor symptoms (hot flushes) during the research. The symptoms were defined as mild, feeling hot with no sweat; moderate, feeling hot with sweating without interfering with activities; severe, feeling hot with sweating that prevents any activities at the time [Citation17]. The hot flush weekly weighted score was calculated according to the severity of hot flushes. We multiplied the number of mild hot flushes by factor one, the number of moderate hot flushes by factor two and the number of severe hot flushes by factor three, weekly [Citation11].
The secondary efficacy endpoint evaluated the effects of treatment on bone turnover markers in postmenopausal women, vaginal atrophy symptoms and the Vaginal Maturation Index by cytology analysis of vaginal epithelium at baseline and after 12 and 24 weeks. A detailed analysis of the effects on bone turnover markers was published by Costa-Paiva et al. [Citation18].
Evaluation of safety
Clinical (pressure and weight range and vaginal bleeding) and laboratory parameters were included, in addition to the analysis of adverse events during the study and blood pressure was measured at all visits. For anthropometric evaluation, the weight, height and body mass index were measured according to The World Health Organization 2002 criteria.
An evaluation of metabolic safety was performed concerning complete blood count. The evaluation of thyroid-stimulating hormone and glycated hemoglobin was performed only at the beginning of treatment. Evaluations were performed in the morning, before and after 12 and 24 weeks of treatment. Analyses were performed using Sysmex XE-2100D, Variant II Turbo–Biorad, Modular E170, Centaur and Advia 2400 equipment.
Safety was also assessed by analyzing the data obtained in clinical, physical examination, blood pressure measurement, weight, gynecological examination and laboratory tests, mammography, cervical pap smear transvaginal ultrasound and the analysis of the adverse events occurring during the study.
From the notes in the participant diary, a standard analysis was conducted for bleeding. This analysis comprised the presence or absence of bleeding or menstrual spotting and its duration during treatment, comparing both intervention groups. Bleeding was defined as the presence of vaginal bleeding requiring the use of a sanitary napkin and spotting as vaginal bleeding not requiring the use of a sanitary napkin (except for a panty liner).
Statistical analysis
The sample size calculation was based on the study by Panay et al. that showed a 29% reduction in the frequency of vasomotor symptoms between baseline and after 12 weeks of treatment with the combination of 0.5 mg 17β-E2/0.1 mg NETA when compared to placebo [Citation14]. A standard deviation of 40% for the difference between the groups was assumed, based on the results seen by Notelovitz and Mattox [Citation17]. Thus, considering the difference between values, for a coefficient of 80% and an error margin of 5%, the sample size was estimated as at least 31 women per group. Considering the loss to follow-up of approximately 50%, the sample size used was 60 women per group. The statistical analysis method used was the intention to treat (ITT).
Variables were assessed for distribution normality by the Shapiro–Wilk test and for homogeneity by the Levene test. For data analysis, the mean and standard deviation were calculated for quantitative variables and the frequency and percentage for categorical variables. For variables with normal distribution, the paired t-test was used to compare values within the groups and the independent t-test was used to compare means from independent groups. Categorical variables were compared using the chi-square test and the exact Fisher test. The Mann–Whitney U-test and the Wilcoxon test were conducted for non-parametric variables. In all tests, a significance level of 5% or the corresponding p-value was used. The analyses were conducted using Statistical Analysis System (SAS) software, version 9.2.
Results
A total of 118 women were evaluated, at a mean age range of 53 years, who were predominantly white, with no differences in demographics or clinical characteristics between the groups ().
Table 1. Demographic and clinical baseline characteristics of participants according to treatment group.
A significant reduction was seen in the frequency of vasomotor symptoms by the end of six treatment cycles with the combination of an ultra-low dose of 0.5 mg 17β-E2/0.1 mg NETA (). HT therapy users had a 77.1% decrease in the mean frequency of hot flushes to treatment start. On the other hand, the placebo group had a reduction of 49.9%. The difference was statistically significant (p = 0.0001).
Figure 2. Number of hot flushes per week before and at the end of treatment (intention-to-treat [ITT] analysis population). 17β-E2, 17β-estradiol; NETA, norethisterone acetate.
![Figure 2. Number of hot flushes per week before and at the end of treatment (intention-to-treat [ITT] analysis population). 17β-E2, 17β-estradiol; NETA, norethisterone acetate.](/cms/asset/59242b19-7c7e-4f21-9dfa-9192ef16956e/icmt_a_2190507_f0002_b.jpg)
After 4, 8, 12 and 24 weeks from the treatment start, the frequency of hot flushes was significantly lower in the treatment group when compared to the placebo (p < 0.0001; ).
Figure 3. Mean number of hot flushes by week in both groups (0.5 mg 17β-estradiol [17β-E2]/0.1 mg norethisterone acetate [NETA] and placebo group). Intention-to-treat (ITT) analysis population.
![Figure 3. Mean number of hot flushes by week in both groups (0.5 mg 17β-estradiol [17β-E2]/0.1 mg norethisterone acetate [NETA] and placebo group). Intention-to-treat (ITT) analysis population.](/cms/asset/5e1c45de-5844-479b-a94b-e501b07f80c9/icmt_a_2190507_f0003_b.jpg)
In the analysis of the severity score of vasomotor symptoms, the percentage change in the severity of hot flushes throughout the study in the ITT analysis population, a significant reduction was seen in the treatment group when compared to placebo (p < 0.0001), where the users of HT showed significantly higher variation means than the placebo group ( and ).
Figure 4. Percentage change of hot flush weekly weighted score (HFWWS) at weeks 4, 8, 12 and 24 of treatment compared to baseline. Intention-to-treat (ITT) analysis population. HFWWS = (mild hot flushes × 1) + (moderate hot flushes × 2) + (severe hot flushes × 3). 17β-E2, 17β-estradiol; NETA, norethisterone acetate.
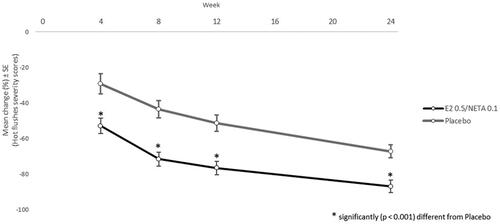
Table 2. Severity score of vasomotor symptoms before and at end of treatment (intention-to-treat [ITT] analysis population): hot flush weekly weighted score.
The significant effect of treatment response with 0.5 mg 17β-E2/0.1 mg NETA may also be shown, where increased range means were seen at weeks 4, 8 and 12 when compared to baseline (p < 0.0001 for all comparisons), increased range means were seen at weeks 8 and 12 when compared to week 4 (p = 0.0343 and p < 0.0001, respectively) and increased range mean was seen at week 12 when compared to week 8 (p < 0.0001).
Regarding the bleeding pattern, most women did not show any bleeding in every cycle. The amenorrhea rate (absence of bleeding and/or spotting) for treatment and placebo groups was 84.0% and 80.0%, respectively, for cycle 1; 86.4% and 88.2% for cycle 2; 79.2% and 77.6% for cycle 3; 85.1% and 86.0% for cycle 4; 78.7% and 92.0% for cycle 5; and 85.4% and 85.7% for cycle 6.
The clinical parameters – weight, blood pressure and gynecological aspects, such as vaginal and breast examinations – were maintained stable throughout the evaluation period, as well as the evaluated laboratory parameters. Adverse events were reported in 28 women (49.1%) in the 17β-E2/NETA group and 26 (43.3%) in the placebo group. Most adverse events were mild and not related to the medication. Adverse events that were experienced at a frequency above 5% were headache, breast tenderness, increased vaginal bleeding and hypercholesterolemia (see Supplementary material). Two participants experienced severe adverse effects, with one case of cholecystectomy in the treatment group and one case of acute cholecystitis in the placebo group, both with full recovery.
Discussion
According to the initial proposal, superiority was shown for the combination of 0.5 mg 17β-E2 and 0.1 mg NETA in a continuous ultra-low-dose combination regimen by oral route in the reduction of vasomotor symptom frequency. It is worth emphasizing that a placebo has a non-negligible effect to reduce the frequency of vasomotor symptoms, which is a well-known effect [Citation6]; however, the active treatment was shown to be more effective, with greater symptom reduction. It should be mentioned that this is the first study with this ultra-low-dose regimen in Brazilian postmenopausal women and that it confirms the efficacy seen in other studies [Citation11,Citation14].
An important point to be taken into consideration is that the observational period of the frequency of vasomotor symptoms was higher at baseline (2 weeks) than at the end of treatment (1 week). This could have caused bias in the results. Thus, a secondary analysis was performed where the corrected frequency of vasomotor symptoms was considered for 1 week, demonstrating a greater reduction of vasomotor symptoms with HT, with a decrease of 77.1% in the ITT population and 79.8% in the per-protocol population, versus a reduction of 49.9% and 55.4%, respectively, in the placebo group.
Although this is an ultra-low-dose regimen, the effect is fast. Panay et al. found statistical significance in the reduction from week 3 [Citation14]. In this study, we noted efficacy from week 4, our first time point for symptom accountability. That is, the superiority was already seen versus placebo in the first treatment cycle.
Here, two analyses were also conducted: one considering the frequency of symptoms within 2 weeks before treatment, and the other considering the average of 2 weeks (i.e. by correcting the observational period to be comparable to the end of treatment). Both analyses showed symptom reduction in the first treatment cycle.
The reduction of vasomotor symptom frequency is important, but not sufficient; therefore, there is a need to assess symptom severity as a secondary efficacy parameter in this study. The results of our study are in line with the CHOICE study, which showed a reduction in the frequency of moderate and severe hot flushes with ultra-low-dose HT [Citation14]. Moreover, Notelovitz et al. compared several doses of oral 17β-E2 to placebo and noted that 0.5 mg was the lowest effective dose for the relief of vasomotor symptoms [Citation11].
The results showed the efficiency of symptom severity since the first cycle. In the sixth cycle, HT reduced the weighted score of weekly hot flushes to 85.8% in the ITT population and 87.0% in the per-protocol population, versus 67.1% and 70.1%, respectively, for placebo, considered concerning the corrected baseline evaluation.
HT was shown to be safe in this study, with an adverse event rate similar to that in the placebo group (49.1% and 43.3%, respectively), with most events being mild and not related to the medication. What draws attention is the higher rate of vaginal bleeding in the hormone group, but this was expected and is a common adverse effect in hormone treatment of menopause. This was also seen in the study by Sturdee et al. with an identical regimen of HT [Citation15]. It is important to note that, despite this, most women did not experience bleeding and/or spotting in every cycle, with amenorrhea rates varying between 78.7% and 86.4% in the hormone group and from 77.6% to 92.0% in the placebo group. Similarly, Mattsson et al. evaluated the same hormone regimen in postmenopausal women, but through a non-comparative, non-interventional study showed that most women continued to experience amenorrhea [Citation16], analogous to our results.
As the strengths of this study, we can highlight its double-blind, randomized design, the evaluation of vasomotor symptoms both in terms of frequency and severity, and the use of daily diaries to avoid the loss of significant information. On the other hand, some weaknesses may have occurred. If, on the one hand, the use of a daily diary prevents the loss of relevant information, it also requires the participant’s involvement with the study and hampers the participation of women with a lower educational level. As mentioned before, the observational period for the frequency of vasomotor symptoms was greater for baseline (2 weeks) than for the end of treatment (1 week), which could be a reason for bias in the results; however, this was corrected by considering the mean frequency of vasomotor symptoms for 1 week. As limitations of the study, we should also consider the dropout rates and the ITT analysis, which may have influenced some results; however, the results we obtained are in line with the publications available using the same hormone formulation.
Conclusions
Oral HT in a combination of 0.5 mg 17β-E2 and 0.1 mg NETA in a continuous regimen was effective in reducing the frequency and severity of vasomotor symptoms compared to the placebo. Efficacy was demonstrated in week 4 of the treatment.
Potential conflict of interest
A.M.C. is a medical consultant at Libbs Farmacêutica Ltda. The other authors have no potential conflicts of interest to disclose.
Supplemental Material
Download MS Word (31.6 KB)Acknowledgements
The authors would like to thank all participants in this research. Medical support for this manuscript was provided by Libbs Farmacêutica Ltda, Brazil.
Additional information
Funding
References
- Baber RJ, Panay N, Fenton A. IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric. 2016;19(2):109–150.
- de Villiers TJ, Hall JE, Pinkerton JV, et al. Revised global consensus statement on menopausal hormone therapy. Maturitas. 2016;91:153–155.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Pompei L, Machado R, Wender M, et al. Consenso Brasileiro de Terapêutica Hormonal da Menopausa – Associação Brasileira de Climatério (SOBRAC). Leitura Médica. 2018.
- Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am. 2015;44(3):497–515.
- Maclennan AH, Broadbent JL, Lester S, et al. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev. 2004;18(4):CD002978.
- Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291(13):1610–1620.
- Ettinger B. Rationale for use of lower estrogen doses for postmenopausal hormone therapy. Maturitas. 2007;57(1):81–84.
- Langer RD. Efficacy, safety, and tolerability of low-dose hormone therapy in managing menopausal symptoms. J Am Board Fam Med. 2009;22(5):563–573.
- Gambacciani M, Biglia N, Cagnacci A, et al. Menopause and hormone replacement therapy: the 2017 recommendations of the Italian menopause society. Minerva Ginecol. 2018;70(1):27–34.
- Notelovitz M, Lenihan JP, McDermott M, et al. Initial 17beta-estradiol dose for treating vasomotor symptoms. Obstet Gynecol. 2000;95(5):726–731.
- Johansen OE, Qvigstad E. Rationale for low-dose systemic hormone replacement therapy and review of estradiol 0.5 mg/NETA 0.1 mg. Adv Therapy. 2008;25(6):525–551.
- Stute P, Becker HG, Bitzer J, et al. Ultra-low dose – new approaches in menopausal hormone therapy. Climacteric. 2015;18(2):182–186.
- Panay N, Ylikorkala O, Archer DF, et al. Ultra-low-dose estradiol and norethisterone acetate: effective menopausal symptom relief. Climacteric. 2007;10(2):120–131.
- Sturdee DW, Archer DF, Rakov V, et al. Ultra-low-dose continuous combined estradiol and norethisterone acetate: improved bleeding profile in postmenopausal women. Climacteric. 2008;11(1):63–73.
- Mattsson L, Ipsen HE, Granqvist CJ, et al. Ultra-low-dose estradiol and norethisterone acetate: bleeding patterns and other outcomes over 52 weeks of therapy. Climacteric. 2015;18(3):419–425.
- Notelovitz M, Mattox JH. Suppression of vasomotor and vulvovaginal symptoms with continuous oral 17beta-estradiol. Menopause. 2000;7(5):310–317.
- Costa-Paiva L, O Wender MC, Machado RB, et al. Effects of ultra-low dose hormone therapy on biochemical bone turnover markers in postmenopausal women: a randomized, placebo-controlled, double-blind trial. Post Reprod Health. 2022;28(3):149–157.