Abstract
In experimental diabetes, enzymes of glucose and fatty acid metabolism are markedly altered. We investigated the effect of Scoparia dulcis. L. plant extract on hepatic key metabolic enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Scoparia dulcis. plant extracts (SPEt) (aqueous, ethanol and chloroform) were orally administered at doses of 50, 100 and 200 mg/kg body weight respectively for 3 weeks, after which hexokinase, glucose 6-phosphate dehydrogenase, glucose 6-phosphatase, fructose 1,6-bisphosphatase in liver and glycogen in liver and muscle were assayed. Blood glucose, glucose 6-phosphatase and fructose 1,6-bisphosphatase were significantly increased and plasma insulin, hexokinase, glucose 6-phosphate dehydrogenase and glycogen were significantly decreased in diabetic rats. Diabetic rats treated with SPEt significantly reversed all these changes to near normal. Aqueous extract of Scoparia dulcis. was comparatively better than ethanol and chloroform extracts. These results indicate that the aqueous extract of Scoparia dulcis. showed antihyperglycaemic effect by attenuating the above biochemical alterations in streptozotocin diabetes.
Latin binomials:
Cassia auriculata. L.
Coccinia indica. L.
Phaseolus vulgaris. L.
Syzigium cumini. L.
Introduction
According to recent estimates, the human population worldwide appears to be in the midst of a diabetes epidemic (Hu et al., Citation2003). The World Health Organization (WHO) predicts that the number of cases worldwide for diabetes, now at 150 million, will double by 2005 (Marx, Citation2002). Diabetes mellitus is characterized by chronic hyperglycaemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action or both (Baquer et al., Citation1998). The liver plays a pivotal role in glucose and lipid homeostasis (Gupta et al., Citation1999). In experimental diabetes, enzymes of glucose metabolism are markedly altered and produce hyperglycaemia, which leads to pathogenesis of diabetic complication (Sochar et al., Citation1985). Despite the great strides that have been made in the understanding and management of diabetes, the disease and disease related complications are increasing unabated (Tiwari & Madhusudana Rao, Citation2002). Inspite of the presence of known antidiabetic medicine in the pharmaceutical market, remedies from medicinal plants are used with success to treat this disease (Bhattaram, Citation2002).
Scoparia dulcis. L. (Scrophulariaceae) is a perennial herb widely distributed in tropical and subtropical regions. In these regions, the fresh or dried plant of Scoparia dulcis. has traditionally been used as one of the remedies for stomach troubles (Satyanarayana, Citation1969), hypertension (Chow et al., Citation1974), diabetes (Perry, Citation1980), inflammation (Gonzales Torres, Citation1986), bronchitis (Farias Freie et al., Citation1993) hemorrhoids and hepatosis (Satyanarayana, Citation1969) and as an analgesic and antipyretic (Gonzales Torres, Citation1986). A number of different principles, include scoparic acid A, scoparic acid B (Hayashi et al., Citation1993), scopadulcic acid A and B, scopadulciol (Hayashi et al., Citation1990) and scopadulin (Hayashi et al., Citation1991), have been identified as contributors to the observed medicinal effect of the plant. These compounds were found to possess various biological activities such as inhibitor against replication of Herpes simplex virus, gastric H+, K+ ATPase activator and antitumor promoting activity, etc. In a previous study, Nath (Citation1943) studied the antidiabetic effect of Scoparia dulcis. and obtained a glycoside, ammelin from fresh plant and reported that it brought relief in other ailments accompanied with diabetes (i.e., pyorrhoea, eye troubles, joint pain, susceptibility to cold, etc.) within a very short period.
To our knowledge, there are no available reports on the effect of Scoparia dulcis. on enzymes of hepatic glucose metabolism in streptozotocin-induced diabetes. Therefore, the present study investigates the effect of Scoparia dulcis. extracts (aqueous, ethanol and chloroform) on hepatic key enzymes in male albino Wistar rats.
Materials and Methods
Animals
Male albino Wistar rats, body weight of 180–200 g bred in Central Animal House, Rajah Muthiah Medical College, Annamalai University, were used in this study. The animals were fed a pellet diet (Hindustan Lever Ltd., India) and water ad libitum.. The animals used in the present study were maintained in accordance with the guidelines of the National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India and approved by the ethical committee (Vide. No: 73, 2002), Annamalai University. Animals were maintained under a constant 12 h light and dark cycle and an environmental temperature of 21–23°C.
Drugs and chemicals
All the drugs and biochemicals used in this experiment were purchased from Sigma Chemical Company, St. Louis, MO, USA. The chemicals were of analytical grade.
Plant material
Scoparia dulcis. L. plants were collected from Neyveli, Cuddalore District, Tamil Nadu, India. The plant was identified and authenticated at the Herbarium of Botany Directorate in Annamalai University. A voucher specimen (No. 3412) was deposited in the Botany Department of Annamalai University.
Preparation of Scoparia Dulcis. Plant extract (SPEt)
Aqueous and chloroform extracts
Scoparia dulcis. fresh whole plants (500 g) were extracted with 1.5 L of water/chloroform by the method of continuous hot extraction. The filtrate was evaporated to constant weight on a rotavapor. The residual extract was dissolved in sterile water and used in the investigation (Jain, Citation1968).
Ethanol extract
Fresh plant of Scoparia dulcis. (500 g) was chopped into small pieces and soaked overnight in 1.5 L of 95% ethanol. This suspension was filtered and the residue was resuspended in an equal volume of 95% ethanol for 48 h and filtered again. The two filtrates were pooled and the solvents were evaporated in a rotavapor at 40–50°C under reduced pressure and lyophilized. A greenish-black powdered material was obtained (20–30 g). It was stored at 0–4°C until needed, and then the residual extract was suspended in 1.0 ml of distilled water and used in the study (Hossain et al., Citation1992).
Induction of experimental diabetes
A freshly prepared solution of streptozotocin (45 mg/kg) in 0.1 M citrate buffer, pH 4.5 was injected i.p. in a volume of 1 ml/kg (Siddique et al., Citation1987). Fourty-eight hours after streptozotocin administration, rats with moderate diabetes having glycosuria and hyperglycaemia (i.e., with blood glucose of 200–300 mg/dl) were taken for the experiment.
Experimental design
In the experiment, a total of 66 rats (60 diabetic surviving rats, 6 normal rats) were used. The rats were divided into 11 groups of 6 rats each. Three doses of aqueous, ethanol and chloroform extracts (50, 100 and 200 mg/kg per day) were tested. All doses were started 48 h after streptozotocin injection. Blood samples were drawn at weekly intervals until the end of study (i.e., 3 weeks). At the end of the 3rd week, all the rats were killed by decapitation (pentobarbitone sodium) anesthesia (60 mg/kg). Blood was collected in tubes containing potassium oxalate and sodium fluoride solution for the estimation of blood glucose and plasma was separated for assay of insulin. Liver and muscle were dissected out, washed in ice-cold saline, patted dry and weighed.
Analytical procedure
Glucose levels were estimated by the O.-toluidine method of Sasaki et al., (Citation1972). Plasma insulin was assayed with an ELISA kit (Boeheringer-Manneheim Kit, Manneheim, Germany). Hepatic hexokinase was assayed by the method of Brandstrup et al. (Citation1957). Glucose 6-phosphatase and fructose 1,6-bisphosphatase activities were measured by phosphate released by the methods of Baginsky et al. (Citation1974) and Gancedo and Gancedo (Citation1971). The colorimetric determination of phosphorous in the supernatant was estimated by the method of Fiske and Subbarow (Citation1925).
Glucose 6-phosphate dehydrogenase was assayed by the method of Ellis and Kirkman (Citation1961). Liver and muscle glycogen was estimated by the method of Morales et al., (Citation1973). Protein was determined by following the method of Lowry et al. (Citation1951).
Statistical analysis
All data were expressed as mean±S.D of number of experiments (n = 6). The statistical significance was evaluated by one-way analysis of variance (ANOVA) using SPSS version 7.5 (SPSS, Cary, NC, USA) and the individual comparison were obtained by Duncans' Multiple Range Test (DMRT) (Duncan, Citation1957).
Results
In all groups prior to streptozotocin administration, the basal levels of blood glucose of the rats were not significantly different. However, 48 h after streptozotocin administration, blood glucose levels were significantly higher in rats selected for the study. In contrast, non-diabetic controls remained persistently euglycaemic throughout the course of the study.
shows the effect of treatment with extracts on blood glucose levels. Although a significant antihyperglycemic (p < 0.01) effect was evident from first week onwards, decrease in blood sugar was maximum on completion of the third week (66.53%) (p < 0.001) in the group receiving 200 mg/kg/day of aqueous extract of Scoparia dulcis.. On the other hand, ethanol and chloroform extracts treated groups showed an antihyperglycaemic much later (i.e., on completion of the third week) in groups receiving 200 mg/kg day (60.53 and 56.31%, respectively). On the basis of these studies, doses of 200 mg/kg per day of aqueous, ethanol and chloroform extracts of Scoparia dulcis. were selected for further evaluation.
Table 1.. Effect of 3-week treatment with various doses of aqueous, ethanolic and chloroform extracts of Scoparia dulcis. on glucose in normal and experimental rats.
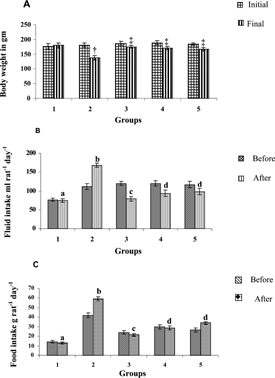
The body weights of SPEt treated groups were increased significantly (p < 0.001) on the 3rd week compared with diabetic control rats (). The food intake was significantly lowered in the SPEt treated groups (p < 0.001) when compared with the vehicle treated group. Similarly, the water intake was significantly reduced (p < 0.001) in SPEt treated groups.
Figure 1. Body weight, fluid and food intake in streptozotocin diabetic rats before and after oral treatment with Scoparia dulcis. plant extract for 3 weeks. Groups: 1-Normal; 2-Diabetic control; 3-Diabetic + Aq-SPEt (200 mg/kg body weight); 4-Diabetic + Alc-SPEt (200 mg/kg body weight); 5-Diabetic + Chloro-SPEt (200 mg/kg body weight). Values are given as mean ± S.D from six rats in each group. Values not sharing a common superscript letter differ significantly at p < 0.05 (DMRT). Duncan Procedure; Ranges for the level: 2.95; 3.09; 3.20, 3.22 Diabetic control was compared with normal, †p < 0.001. Experimental groups were compared with diabetic control ‡p < 0.001.
represents the effect of Scoparia dulcis. on plasma insulin and glycogen content in liver and muscle of normal and experimental animals. In diabetic controls, plasma insulin and hepatic and skeletal muscle glycogen content were decreased significantly as compared to non-diabetic controls. Treatment with SPEt increased the plasma insulin, hepatic and skeletal glycogen significantly. The extent of increase was higher in groups treated with aqueous extract of Scoparia dulcis. than ethanol and chloroform extracts treated groups.
Table 2.. Changes in the levels of plasma insulin and tissue glycogen in normal and experimental animals.
depicts the activities of carbohydrate metabolizing enzymes in liver of normal and SPEt treated diabetic rats. The activities of enzyme hexokinase and glucose 6-phosphate dehydrogenase were found to be decreased whereas the activities of gluconeogenic enzymes: glucose 6-phosphatase and fructose 1,6-bisphosphatase were significantly increased in diabetic rats. SPEt administration to diabetic rats reversed the above changes in a significant manner when compared to diabetic rats.
Table 3.. Changes in the activities of hepatic hexokinase, glucose 6-phosphate dehydrogenase, glucose 6-phosphatase and fructose 1,6-bis phosphatase in normal and experimental rats.
Discussion
Global estimates suggest that three fourths of the world population cannot afford the products of allopathic medicine and, thus, have to rely upon the use of traditional medicines, which are largely derived from plants (Hu et al., Citation2003). The present study was undertaken to assess the antihyperglycaemic property of Scoparia dulcis., which have been reported in Ayurveda to be useful in treatment of diabetes mellitus. In the present study, treatment with aqueous, ethanol and chloroform extracts of Scoparia dulcis. showed significant antihyperglycaemic activity. The maximum reduction in glucose levels was seen in groups receiving 200 mg/kg of the three extracts respectively. This is probably indicative of efficacy of the plant. Moreover, it indirectly indicates that part of the antihyperglycaemic activity of this plant is through release of insulin from the pancreas. In this context a number of other plants have also been reported to have antihyperglycaemic and insulin-release stimulatory effects (Venkateswaran and Pari, Citation2002a; Latha & Pari, Citation2003). The daily administration of SPEt to streptozotocin diabetic rats for 3 weeks caused a statistically significant reduction in food and fluid intakes and an increase in the body weight. This could be the result of improved glycemic control produced by Scoparia dulcis..
In the present study, hepatic and skeletal muscle glycogen content was reduced significantly in diabetic controls. Administration of SPEt prevented the depletion of glycogen content but could not normalize it. This prevention is due to stimulation of insulin release from β-cells or due to insulinomimetic activity of some component of plant resulting in direct peripheral glucose uptake.
Hexokinase insufficiency in diabetic rats can cause decreased glycolysis and decreased utilization of glucose for energy production (Vats et al., Citation2003). Administration of SPEt to diabetic rats enhanced the hexokinase activity and suggests greater uptake of glucose from blood by liver cells and increased glycolysis.
The activity of glucose 6-phosphate dehydrogenase decreased in the present study. The decrease in the activity of this enzyme in diabetic condition may result in the diminished functioning of HMP shunt and thereby the production of reducing equivalent such as NADH and NADPH (Weber & Convery, Citation1966; Saraswathi panneerselvam & Govindaswamy, Citation2002). In our study, administration of SPEt increased the activity of glucose 6-phosphate dehydrogenase considerably. This may be attributed to the insulin secretory effect of Scoparia dulcis., as glucose 6-phosphate dehydrogenase has been reported to increase the supply of NADPH.
Increased activity of glucose 6-phosphatase in diabetic rats provide hydrogen which binds with NADP+ in the form of NADPH and enhances synthesis of fats from carbohydrates, i.e., lipogenesis (Bopanna et al., Citation1997) and finally contribute to increased levels of glucose in blood. Increased hepatic glucose production in diabetes mellitus is associated with impaired suppression of the gluconeogenic enzyme fructose 1,6-bisphosphatase. In the diabetic state, several workers have observed increased activities of gluconeogenic enzymes (Sochar et al., Citation1985; Prince et al., Citation1997; Venkateswaran & Pari, Citation2002b). Activation of gluconeogenic enzymes is due to state of insulin deficiency since under normal condition insulin function as a suppressor of gluconeogenic enzymes.
Administration of SPEt significantly depressed the activities of gluconeogenic enzymes in diabetic rats. The level of plasma insulin was found to increase significantly in diabetic rats treated with SPEt, which may be a consequence for the significant reduction in the level of gluconeogenic enzymes. The reduction in the activities of gluconeogenic enzymes can result in the decreased concentration of glucose in blood.
The present data on the effect of Scoparia dulcis. extract on streptozotocin-induced diabetic rats indicates that impairment in the glucose metabolizing enzymes in liver have been corrected by the insulin secretory effect and by the presence of active constituents such as glycosides – ammelin and diterpenoids – scoparic acids and scopadulcic acids. Although ethanol and chloroform extracts produced antihyperglycaemic activity, the effect was more pronounced in aqueous extract. Detection of antihyperglycaemic activity in SPEt along with protective effect against streptozotocin challenge provides a scientific rationale of use of Scoparia dulcis. as an antidiabetic plant.
Acknowledgement
The authors wish to thank the University Grants Commission, New Delhi Project No.F.12–36/2001 (SR-I) for the research grant and a research fellowship to Ms. M. Latha.
References
- Baginsky ES, Foa PP, Zak B (1974): Glucose-6-phosphatase. In: Bergymeyer HU, ed., Methods of Enzymatic Analysis, 2nd edition Academic Press, New York, Vol 2, p 788–792.
- Baquer NZ, Gupta D, Raju J (1998): Regulation of metabolic pathways in liver and kidney during experimental diabetes: Effects of antidiabetic compounds. Ind J Clin Biochem 13: 63–80.
- Bhattaram VA, Ceraefe M, Kohlest C, Vest M, Deundorf H (2002): Pharmacokinetics and bioavailabitlity of herbal medicinal products. Phytomedicine 9 (Suppl III): 1–36.
- Bopanna KN, Kannan J, Sushma G, Balaraman R (1997): Antidiabetic and antihyperlipidemic effects of Neem seed kernel powder on alloxan diabetic rabbits. Ind J Pharmacol 29: 162–167.
- Brandstrup N, Kirk JE, Bruni C (1957): Determination of hexokinase in tissues. J Gerontol 12: 166–171.
- Chow SY, Chen SM, Yang CM, Hsu H (1974): Pharmacological studies on China herbs (I) Hypotensive effect of 30 chinese herbs. J Formosan Med Assoc 73: 729–739.
- Duncan BD (1957): Multiple range tests for correlated and heteroscedastic means. Biometrics 13: 359–364.
- Ellis HA, Kirkman HN (1961): A Colorimetric method for assay of erythrocyte gluocse-6-phosphate dehydrogenase. Proc Soc Exp Biol Med 106: 607–609.
- Farias Freie SM, Silva Emin JA, Lapa AJ, Souccar C, Brandao Torres LM (1993): Analgesic and anti-inflammatory properties of Scoparia dulcis. L. extract and glutinol in rodents. Phytother Res 7: 408–414.
- Fiske CH, Subbarow J (1925): The colorimetric determination of phosphorus. J Biol Chem 66: 375–400.
- Gancedo JM, Gancedo C (1971): Fructose-1,6-diphosphatase, phospho-fructokinase and glucose-6-phosphate dehydrogenase from fermenting and non-fermenting yeasts. Arch Microbiol 76: 132–138.
- Gonzales Torres DM (1986): Cataogo de plantas medicinales (y alimenticias y utiles) Usada en paraguay, Asuncion, Paraguay, pp 394.
- Gupta D, Raju J, Prakash JR, Baquer NZ (1999): Changes in the lipid profile, lipogenic and related enzymes in the liver of experimental diabetic rats: Effect of insulin and vanadate. Diab Res Clin Pract 46: 1–7. [CROSSREF], [CSA]
- Hayashi T, Kawaski M, Miwa Y, Taga T, Morita N (1990): Antiviral agents of plant origin III. Scopadulin, a novel tetracyclic diterpene from Scoparia dulcis. L. Chem Pharm Bull 38: 945–947.
- Hayashi T, Asano S, Mizutani M, Takeguchi N, Okamura K, Morita N (1991): Scopadulciol, an inhibitor of gastric H+, K+-ATPase from Scoparia dulcis. and its structure activity relationships. J Nat Prod 54: 802–809. [CROSSREF]
- Hayashi T, Okamura K, Tamada Y, Iida A, Fujita T, Morita N (1993): A new chemotype of Scoparia dulcis.. Phytochemistry 33: 349–352. [CROSSREF]
- Hossain, MZ, Shibib BA, Rahman R (1992): Hypoglycemic effects of Coccinia indica.: Inhibition of key gluconeogenic enzyme, glucose-6-phosphatase. Ind J Exp Biol 10: 418–420.
- Hu X, Sato J, Oshida Y, Yu M, Bajotto G, Sato Y (2003): Effect of Gosha-jinki-gan (Chinese herbal medicine): (Niu-che-sen-qi-wan) on insulin resistance in streptozotocin induced diabetic rats. Diab Res Clin Pract 59: 103–111. [CROSSREF], [CSA]
- Jain SR (1968): Hypoglycaemic principle in the Musa sapientum. and its isolation. Plant Med 1: 43–47.
- Latha M, Pari L (2003): Preventive effects of Cassia auriculata. L. flowers on brain lipid peroxidation in rats treated with streptozotocin. Mol Cell Biochem 243(1–2): 23–28.
- Lowry OH, Roesborough MJ, Farr AL, Randall RJ (1951): Protein measurement with Folin-phenol reagent. J Biol Chem 193: 265–275.
- Marx J (2002): Unravelling the causes of diabetes. Science 296: 686–689. [CROSSREF]
- Morales MA, Jabbagy AJ, Tenenzi HP (1973): Mutations affecting accumulation of glycogen. Neurospora News Lett 20: 24–25.
- Nath MC (1943): Investigations on the new antidiabetic principle (amellin) occurring in nature Part I. Studies on some of its biochemical properties. Ann Biochem Exp Med 3: 55–62.
- Perry LM (1980): Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses. The MIT Press, Cambridge, pp 385.
- Prince PSM, Menon VP, Pari L (1997): Effect of Syzigium cumini. extracts on hepatic hexokinase and glucose-6-phosphatase in experimental diabetes. Phytother Res 11: 529–531. [CROSSREF]
- Saraswathi Panneerselvam R, Govindaswamy S (2002): Effect of sodium molybdate on carbohydrate metabolizing enzymes in alloxan-induced diabetic rats. J Nut Biochem 13: 21–26. [CROSSREF]
- Sasaki T, Masty S, Sonae A (1972): Effect of acetic acid concentration on the colour reaction in the O.-toluidine boric acid method for blood glucose estimation. Rinshbo kagaku 1: 346–353.
- Satyanarayana K (1969): Chemical examination of Scoparia dulcis. (Linn): Part I. J Ind Chem Soc 46: 765–766.
- Siddique O, Sun Y, Lin JC, Chien YW (1987): Facilitated transdermal transport of insulin. J Pharm Sci 76: 341–345.
- Sochar M, Baquer NZ, Mclean P (1985): Glucose under utilization in diabetes. Comparative studies on the changes in the activities of enzymes of glucose metabolism in rat kidney and liver. Mol Physiol 7: 51–68.
- Tiwari AK, Madhusudana Rao J (2002): Diabetes mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects. Curr Sci 83(1): 30–38.
- Vats V, Yadav SP, Grover JK (2003): Effect of Trigonella foenumgraecum. on glycogen content of tissues and the key enzymes of carbohydrate metabolism. J Ethnopharmacol 85: 237–242. [CROSSREF], [CSA]
- Venkateswaran S, Pari L (2002a): Antioxidant effect of phaseolus vulgaris. in Streptozotocin–induced diabetic rats. Asia Pacific J Clin Nutr 11: 206–209. [CROSSREF], [CSA]
- Venkateswaran S, Pari L (2002b): Effect of Coccinia indica. on blood glucose, insulin and hepatic key enzymes in experimental diabetes. Pharm Biol 40: 165–170.
- Weber G, Convery HJH (1966): Insulin: Inducer of glucose-6-phosphate dehydrogenase. Life Sci 5: 1136–1146.
