ABSTRACT
Aloe ferox. Mill. is one of the plants used for the treatment of sexually transmitted infections (STIs) in the Eastern Cape province of South Africa. Different extracts of the plant were investigated for their antimicrobial constituents. This led to the isolation of three known compounds, namely, 1,8-dihydroxy-3-hydroxymethyl-9,10-anthracenedione (1, aloe-emodin), 1,8-dihydroxy-3-methyl-9,10-anthracenedione (2, chrysophanol), and 10-C.-β-D-glucopyranosyl-1,8-dihydroxy-3-hydroxymethyl-9-anthracenone (3, aloin A). The structures of the compounds were determined by chemical and spectroscopic studies. The antibacterial activity of the compounds (1–3) was demonstrated using the microplate dilution method.
Introduction
Studies in sub-Saharan Africa have shown that more than 70% of the population is affected by sexually transmitted infections (WHO, Citation1995). Plant materials prescribed by traditional healers and herbalists have been used in Africa for the treatment of gonorrhea and syphilis for centuries (Langenhen & Thimann, Citation1982; Amabeoku et al., Citation1998). The use of herbal remedies in the treatment of these diseases is still vital to the provision of primary health care in the continent (Ndubani & Hojer, Citation1999). Aloe ferox. (Asphodelaceace) is widespread in the Eastern Cape province of South Africa. The plant is widely used for the treatment of various diseases including STIs such as gonorrhea and syphilis. The traditional healers of the study area use both fresh and dry leaves of this plant, however, the method of preparation varies from one traditional healer to the other. Whereas some use infusions made from fresh or dried material and taken orally, others squeeze out the juice for direct application on the penile sores. Another method of preparation is pulverization of the leaf, which is mixed with Vaseline to form a paste and is applied topically on the sores.
Extracts from this plant have demonstrated significant activity against bacteria and fungi (Afolayan et al., Citation2002). Although some information is available on the traditional uses of aloe species in herbal medicine (Van Wyk et al., Citation1997) as well as its chemical composition (Speranza et al., Citation1986Citation1990; Koyama et al., Citation1994), no work has been reported on the use of Aloe ferox. for the treatment of STIs. The purpose of this study is to isolate and identify bioactive compounds from this species through bioactivity-guided fractionation of its extract. This is with the view to validate its usage against microbial infections such as the STIs.
Materials and Methods
General experimental procedures
Melting points were determined on a Gallenhamp melting point apparatus and were uncorrected. The UV and IR spectra were recorded on Beckman DU-7400 and Perkin Elmer Fourier-transform infrared (FTIR) spectrometers, respectively. 1H (400 MHz) and 13C (100.60) NMR were recorded on a Bruker AMX 400 instrumentwith chemical shift data reported in ppm relative to the solvent used. 2D NMR spectra were recorded on the same instrument using field gradient BBI (inverse) probe. Mass spectra were recorded on Micromass 70–70E mass spectrometer. FABMS spectra were obtained with m.-nitrobenzyl alcohol matrix. Vacuum liquid chromatography (VLC) and column chromatography (CC) were performed on silica gel 60 H (15 µm) and silica gel (0.063–0.2 mm), respectively. Silica gel F254 60 coated on aluminum plates for thin-layer chromatography (TLC) and silica gel F254 60 coated on glass plates (20 cm × 20 cm) for preparative thin-layer chromatography (PTLC) all were supplied by Merck (Midrand, South Africa). Sephadex LH-20 (25–100 µm) for gel filtration chromatography (GFC) was obtained from Fluka.
Plant material
Leaves of Aloe ferox. were collected from the natural populations around Alice, South Africa, and a voucher specimen (Kambizi Med. Citation2003/1) was deposited at Giffen Herbarium of the University of Fort Hare.
Extraction and isolation
The dried, ground plant material (700 g) was extracted by shaking at room temperature for 3 days. The extract was filtered and evaporated under reduced pressure to give 35.2 g. It was partitioned between n.-hexane and water. The aqueous part was further partitioned between ethyl acetate (EtOAc) and water.
The n.-hexane extract (2.5 g) was fractionated by VLC over silica gel using solvents of increasing polarity (0–100% EtOAc in n.-hexane), and a total of 21 fractions (200 ml each) were collected. The fraction obtained with 45–50% EtOAc in n.-hexane was subsequently subjected to gel filtration (Sephadex LH-20), eluted with CHCl3 followed by MeOH:CHCl3 (5:95). A total of 30 fractions were collected, of which fractions 20–27 showed antimicrobial activity, hence, they were combined. These fractions (0.11 g) were subjected to CC over silica gel using EtOAc:n.-hexane (60:40 v/v). A total of 13 fractions were collected. Yellow fractions 4–11 were combined (70 mg) and further subjected to CC on a silica gel column using 2% MeOH in CHCl3 to give aloe emodin (12 mg).
The EtOAc extract (7.6 g) was subjected to the same fractionation procedure as above using solvent system EtOAc:n.-hexane (0–100%) and then MeOH:EtOAc (0–30%). The eluate obtained from 35% to 40% EtOAc in n.-hexane (0.8 g) was column chromatographed over silica gel and eluted with EtOAc:n.-hexane (60:40 v/v). A total of 49 fractions (20 ml each) were collected. Fractions 11–18 (0.105 g) were combined and further chromatographed over silica gel using EtOAc:n.-hexane (80:20 v/v) to give chrysophanol (20 mg). The fractions eluted from 10% to 15% MeOH in EtOAC were combined (1.3 g), subjected to CC, and eluted with CHCl3:MeOH (90:10 v/v) to give a total of 50 fractions. Fractions 1–7 were combined (0.18 g) and further treated to CC over silica gel using CHCl3:MeOH (90:10 v/v) to yield aloin A (48 mg).
Antibacterial assay
Laboratory strains of Bacillus cereus., Bacillus subtilis., Staphylococcus aureus., Staphylococcus epidermidis., Escherichia coli., and Shigella sonnei. were obtained from the Microbiology Department, Rhodes University, South Africa. During the extraction and purification procedure, bioautographic assay (Slusarenko et al., Citation1989) was performed on TLC plates using B. subtilis.. An inoculated layer of agar was sprayed with fresh culture bacteria over a developed TLC plate and incubated for 24 h at 37°C. As an indicator of bacterial growth, 0.2 mg/ml p.-iodonitrotetrazolium (INT) solution was sprayed over the plate and incubated at 37°C for 30 min. The inhibition of bacterial growth by compounds separated on the TLC plate was visible as white spots against a deep red background.
The MIC values of the pure compounds were determined with microplate dilution method against four Gram-positive (B. cereus., B. subtilis., Staphylococcus aureus., Staphylococcus epidermidis.) and two Gram-negative (E. coli., Shigella sonnei.) bacteria using 96-well microtiter plates. The choice of bacteria strains was to validate the observation made during our previous study (Kambizi & Afolayan, Citation2003) when we reported significant antimicrobial activity of this plant on the same microorganisms. Each test organism was prepared by diluting 24 h old broth culture with sterile nutrient broth. The culture was then diluted 100-fold to give approximately 106 bacteria ml−1. The microtiter plates were prepared using serial dilution (Eloff, Citation1998) and incubated for 24–48 h at 37°C. As an indicator of bacterial growth, 40 µl of 0.2 mg/ml p.-iodonitrotetrazolium solution was added to each well and incubated at 37°C for 30 min. The colorless tetrazolium salt was reduced to a red-colored product by biological activity of the organisms, thereby making the inhibition of bacterial growth visible as clear wells. Minimum inhibitory concentration (MIC) values were recorded as the lowest concentration resulting in complete inhibition of bacterial growth. Each treatment was replicated three times. Streptomycin, chloramphenicol, solvents, and sample-free solutions were used as standard and blank controls.
Results and Discussion
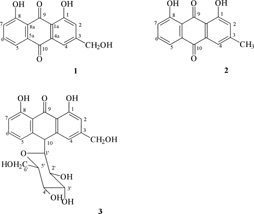
Aloe ferox. is one of the most frequent and common plants used by the community for the treatment of STIs. Three compounds; 1,8-dihydroxy-3-hydroxymethyl-9,10-anthracenedione (1, aloe emodin), 1,8-dihydroxy-3-methylanthracenedione (2, chrysophanol), and 10-C.-β-D-glucopyranosyl-1,8-dihydroxy-3-hydroxymethyl-9-anthracenone (3, aloin A) () were isolated from the leaves of this species. Although not novel, chrysophanol was isolated from this plant for the first time. Aloe emodin showed inhibitory activity against all tested organisms with MIC ranging from 62.5 µg/ml in B. subtilis. to 250 µg/ml in S. epidermidis., and Shigella sonnei. (). Chrysophanol was active against B. subtilis., S. epidermidis., and E. coli. while aloin A inhibited all the test bacterial strains.
Table 1.. Antibacterial activity of compounds isolated from Aloe ferox..
Hatano et al. (Citation1999) reported MICs of aloe emodin and chrysophanol against E. coli. to be >128 µg/ml and 1024 µg/ml, respectively, and the MIC of chrysophanol was 256 µg/ml against methicillin-resistant Staphylococcus aureus.. Aloe emodin has been reported to be an anticancer agent with selective activity against neuroectodermal tumors (Pecere et al., Citation2000). Generally, both aloe-emodin and aloin A have been associated with other biological and medicinal activities that include laxative action (Van Wyk et al., Citation1997). In this study, the activity of the isolated compounds (1–3) against both Gram-positive and Gram-negative bacteria has demonstrated a broad spectrum potential of the plant as an antimicrobial agent. Some of the bacteria used have similar characteristics to those that cause certain STIs. For example, Shigella sonnei. causes shigellosis, a disease transmitted during sexual activity between two men (CDC, Citation2001). All these might have been the reasons for the usage of A. ferox. for the treatment of STIs.
Acknowledgments
The authors are grateful to the NRF, South Africa, the Govan Mbeki Research and Development Centre of the University of Fort Hare, and the Government of Zimbabwe for financial support.
References
- Afolayan AJ, Grierson DS, Kambizi L, Madamombe IT, Masika PJ (2002): In vitro. antifungal activity of some South African medicinal plants. South African J Bot 68: 72–76. [CSA]
- Amabeoku GJ, Leng MJ, Syce JA (1998): Antimicrobial and anticonvulsant activities of Viscum capense.. J Ethnopharmacol 61: 237–241. [CROSSREF], [CSA]
- CDC (2001): Shigella sonnei. outbreak among men who have sex with men. San Francisco, California. M M W R. 42: 922–926.
- Eloff JN (1998): A sensitive and quick microplate method to determine the minimum inhibitory concentration of plant extracts for bacteria. Planta Med 64: 711–713. [CSA]
- Hatano T, Uebayashi H, Ito H, Shiota S, Tsuchiya T, Yoshida T (1999): Phenolic constituents of Cassia. seeds and antibacterial effect of some naphthalenes and anthraquinones on methicillin-resistant Staphylococcus aureus.. Chem Pharm Bull 47: 1121–1127. [CSA]
- Kambizi L, Afolayan AJ (2003): Phytomedicinal studies of four selected medicinal plants used for the treatment of STIs in the Eastern Cape. Fort Hare Papers 12: 10–24.
- Koyama J, Ogura T, Tagahara K (1994): Naphthol [2,3-c.] furan-4,9-dione and its derivatives from Aloe ferox.. Phytochemistry 37: 1147–1148. [CROSSREF]
- Langenhen LH, Thimann KV (1982): Plant Biology and Its Relations to Human Affairs. New York, John Wiley & Sons, pp 1–439.
- Ndubani P, Hojer B (1999): Traditional healers and the treatment of sexually transmitted illness in rural Zambia. J Ethnopharmacol 67: 15–25. [CROSSREF], [CSA]
- Pecere T, Gazzola MV, Mucignat C, Parolin C, Vecchia FD, Cavaggion A, Baso G, Diaspro A, Salvato B, Carli M, Palù G (2000): Aloe emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res 60: 2800–2804.
- Slusarenko AJ, Longland AC, Whitehead IM (1989): A convenient, sensitive and rapid assay for antibacterial activity of phytoalexins. Botanica Helv 99: 203–207. [CSA]
- Speranza G, Dada G, Lunazzi L, Gramatica P, Manitto P (1986): A C.-glucosylated 5-methylchromone from Kenya aloe. Phytochemistry 25: 2219–2222. [CROSSREF]
- Speranza G, Manitto P, Monti D, Lianza F (1990): Ferodoxin, a novel 1-methyltetralin derivative isolated from Cape aloe. Tetrahedron Lett 31: 3077–3080. [CROSSREF]
- Van Wyk BE, Van Oudtshoorn B, Gericke N (1997): Medicinal Plants of South Africa. Briza, Pretoria, pp. 1–304.
- WHO (1995): Resolution, Promotion and Development of Training and Research in Traditional Medicine. WHO Document No. WHA 30:49.