Abstract
Stems, leaves, and flowers of Echinacea purpurea. (L.) Moench (Heliantheae: Asteraceae) were fractionated by various solvents and the fractions evaluated for antiviral activity in relation to chemical composition and distribution within the plant. All of the aqueous fractions contained potent activity against herpes simplex virus and influenza virus. However, although some of this activity could be attributed to polysaccharide and cichoric acid components, their individual contributions could not account for the total antiviral activity; other potent antivirals must be present. In addition, the ethanol- and ethyl acetate–soluble fractions from leaves and stem contained an uncharacterized but potent antiviral photosensitizer, which was absent from the flower extract. None of the fractions, however, contained anti-rhinovirus activity. Thus, part of the alleged benefits of Echinacea purpurea. extracts can be attributed to the presence of anti-influenza and anti-HSV compounds, and some of these activities are likely to be present in various commercial tinctures, teas, capsules, and tablets.
Introduction
Commercial preparations of Echinacea purpurea. (L.) Moench (Heliantheae: Asteraceae) are among the most popular herbal medicines in North America as treatments for colds and flu (Barrett, Citation2003). The major chemical components of E. purpurea. have been well characterized (Bauer, Citation1998), and a number of biological activities have been attributed to some of them; for example, the polysaccharide fraction, which has been shown to stimulate macrophage activity and several functions related to cytokine production (Bauer, Citation1998; Rininger et al., Citation2000; Goel et al., Citation2002; Randolph et al., Citation2003), and groups of phenolic compounds and alkamides, which have demonstrated antiviral and antifungal properties, respectively (Binns et al., Citation2002a; Merali et al., Citation2003). These activities could be related to the reports that some E. purpurea. preparations were able to prevent or control upper respiratory infections (URIs), whereas other preparations did not (Barrett, Citation2003).
In order to determine which components are effective in controlling URIs, we decided to investigate the antiviral properties of various solvent fractions of different aerial parts of E. purpurea. and to compare these activities with the chemical composition of each fraction. E. purpurea. is usually consumed in the form of either an ethanol tincture, which would be enriched in alkamides and polyacetylenes, as well as certain phenolic compounds, or as a tea or other oral form based on water extracts, which would be enriched in polysaccharides and glycoproteins. Therefore, the fractionation procedures were designed to reflect this usage.
Materials and Methods
Echinacea. source
Echinacea purpurea. was commercially grown material sourced from North American growers. Samples were identified according to a recent taxonomic revision (Binns et al., Citation2002b), and voucher specimens were deposited in the herbarium at the University of Ottawa, Canada. Voucher numbers and codes for extracts are provided in .
Table 1a.. Echinacea purpurea. samples voucher numbers and codes for extracts.
Extraction methodology
Ethanol extracts. (70%): Ground dried plant material (10 g) (1 mm Ø screen; Thomas-Wiley mill, Philadelphia, PA, USA) was extracted with an ASE 100 (Accelerated Solvent Extraction, DIONEX) using the following parameters: temperature, 40°C; static time, 5 min; flush volume, 70%; purge time, 100 s; static cycles, 3. These were fractionated according to Binns et al. (Citation2002a) three times with equal parts n.-hexane and distilled water, giving three n.-hexane fractions (pooled into one) and three hydroalcoholic portions (pooled). The latter were fractionated twice with an equal volume of ethyl acetate (pooled), the second of which was adjusted to pH < 2.5 to maximize dissolution of cichoric acid.
Water extracts.: Ground dried plant material (10 g) was extracted twice with 100 ml of dH2O (40°C or 80°C bath for 1 h) and the extracts were filtered.
All fractions were concentrated at 30°C in a rotary-evaporator (not to dryness) and adjusted to 50 ml in the appropriate solvent prior to HPLC separation. The alkamide dodeca-2E.,4E.,8Z.,10E./Z.-tetraenoic acid isobutylamide with peak identification numbers 8 and 9 as assigned by Bauer et al. (Citation1988) (“Tetraene 8/9”) and caffeic acid derivative were analyzed as described previously (Binns et al., Citation2002b).
Cells and viruses
The cell lines.: Vero, H-1 (a subclone of HeLa cells that is particularly sensitive to rhinovirus replication), and BEAS-2B were obtained from American Type Culture Collection (ATCC). They were propagated in Dulbecco MEM with 5–10% fetal bovine serum, without antibiotics or antifungal agents. In the case of BEAS-2B cells, the medium was a 1:1 mix of DMEM and F-12. Herpes simplex virus (HSV) type 1 and influenza virus (FV) were propagated and assayed in Vero cells and H-1, respectively, according to our published methods. Rhinovirus (RV) type 14 was obtained from ATCC. It was propagated and assayed in H-1 cells at 33°C.
Antiviral assays
Complete details of our assay procedures have been given in several recent publications (Binns et al., Citation2002a). The cpe (cytopathic effect) end-point method was used, unless indicated otherwise, in 96-well culture trays. The end point was the highest dilution of extract giving complete elimination of viral cpe produced by 100 infectious units of virus. In some experiments, designed to test for antiviral photosensitizers, the light exposure was omitted (dark antiviral activity) by wrapping the test trays in aluminum foil. Minimum inhibitory concentrations (MICs) were then compared with and without light exposure. In some experiments, plaque assays were used, as indicated in the text, to confirm the results of the cpe end-point methods.
Results
Total herb extracts
shows the fractionation procedure, and the MIC100 values for anti-HSV and anti-FV. The lower numbers represent greater antiviral activity. Both water extracts demonstrated substantial activity against both HSV and FV, but none of the fractions were active against rhinovirus. The ethanol extract was also very active against HSV and FV, as was the ethyl acetate subfraction derived from it. However, the latter was significantly much less active against FV, suggesting the presence of more than one antiviral compound. This was supported by the finding that the ethanol and ethyl acetate fractions contained a strong photosensitizing activity, in contrast with the water fractions, where the activities were only slightly photosensitizing ().
Figure 1. Fractionation scheme for E. purpurea. herb. Numbers in boxes represent MIC100 values (µg/ml) in the antiviral assays. HSV, herpes simplex virus; FV, influenza virus; P/S, presence of photosensitizer.
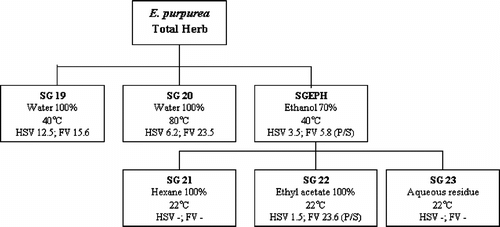
Table 2.. Minimum inhibition concentrations (MIC100) (µg/ml) of total herb fractions from Echinacea purpurea. against herpes simplex virus (HSV) and influenza virus (FV) and presence of photosensitizer (photosens.) are presented. Green tea and red clover were used as negative and positive controls for photosensitizers.
shows the antiviral activities in terms of MICs, based on actual concentrations of each fraction (µg/ml). There were no obvious correlations between activity and relative content of the phenolic compounds (), although those fractions rich in cichoric acid, previously shown to be antiviral (Binns et al., Citation2002a), did possess activity. The ethyl acetate fraction was devoid of the caffeic acid derivatives and contained only a low concentration of alkamides. We cannot therefore identify the active ingredient(s) in this fraction, although we know that it or they must be photosensitizers, and this implicates polyacetylenes/alkamides, some of which are known to possess antiviral activities (Hudson & Towers, Citation1999).
Table 3.. Phytochemical profiling by HPLC of Echinacea purpurea. extracts. The mean concentrations (µg/ml) of caftaric, chlorogenic (chlorog.), caffeic and cichoric acids, as well as cynarin, echinacoside (echin.) and tetraene 8/9 (tet. 8/9) are presented. (Relative Standard Deviation of analyses <5%).
Leaves and stems
The same fractionation procedure was followed for mixed leaves and stems. The antiviral data are shown in as MICs. The results were similar to those for the total herb preparation, and with similar potencies according to the MICs. There was again the distinction between marginal photosensitizing activities of the aqueous fractions and the strong photosensitizing activities in the ethanol and ethyl acetate fractions, suggestive of more than one active compound. No anti-rhinovirus activity was seen.
Table 4.. Minimum inhibition concentration (MIC100) (µg/ml) of leaves and stem fractions from Echinacea purpurea. against herpes simplex virus simplex virus (HSV) and influenza virus (FV) and presence of photosensitizer (photosens.) are presented.
In general, a rough correlation could be drawn between overall phenolic content with antiviral activities, but not with any individual compound, although again cichoric acid was dominant in the most active fractions.
Flower extracts
A different picture emerged from the results of the flower fractions. Data are expressed in as MICs. The water fractions were as potent as the previous extracts (all of these fractions were assayed simultaneously; thus relative potencies are real and were reproducible) and did not appear to contain photosensitizers. In contrast, the ethanol and ethyl acetate fractions only contained marginal activity, which could represent traces of the active ingredient found in the leaves and stems. However, this activity in the flowers was too low to be able to evaluate for photosensitizing activity.
Table 5.. Minimum inhibition concentration (MIC100) (µg/ml) of flower fractions from Echinacea purpurea. against herpes simplex virus (HSV) and influenza virus (FV) and presence of photosensitizer (photosens.) are presented.
All of these fractions were particularly rich in the phenolic compounds; but clearly they did not correlate with antiviral activity. Some of these compounds, including cichoric acid, are known to contribute to the antiviral activities, but evidently other ingredients also contribute.
Polysaccharide fractions
A polysaccharide-enriched fraction (SG-34) was evaluated for antiviral activity, along with purified fractions derived from it. Only the crude fraction contained significant antiviral activity against HSV (MIC = 62 µg/ml) and FV (MIC = 125 µg/ml), but none against rhinovirus. This activity was only moderate, however, in comparison with the fractions documented above, and this suggests that only a part of the activities attributed to the aqueous fractions could be due to polysaccharide or glycoprotein components.
Discussion
It is evident from these results that Echinacea purpurea. extracts contain several distinct antiviral activities, as shown by the ability of various fractions and subfractions to inactivate herpes simplex virus (HSV) and influenza virus (FV). However, none of them were active against rhinovirus (RV). HSV and FV have similar membrane structures, whereas RV does not possess a membrane. This suggests a possible membrane target for the antiviral ingredients. This also implies that if such preparations are really beneficial in the prevention or treatment of colds and flu, then indirect antiviral activities, such as immune-modulating activities (Baron et al., Citation2000; Randolph et al., Citation2003), must be responsible for counteracting rhinovirus colds. This possibility is under study in our laboratory.
Leaf/stem extract and total herb extract gave similar antiviral profiles among the different fractions. Both aqueous and ethanol fractions contained similar activities against the two viruses, but in the case of the ethanol fraction, and its ethyl acetate subfraction, at least part of the activity was due to one or more photosensitizers, in contrast with the water-soluble activity, which showed little or no photosensitizing activity. This is not unusual, as we have found in many of our studies on herbal medicines that photosensitizers, such as thiophenes, alkaloids, and complex quinones, are often responsible for direct antiviral activities, and we have previously demonstrated bio-active photosensitizing polyacetylenes and alkamides in members of the Asteraceae (Towers et al., Citation1997; Hudson & Towers, Citation1999).
Some of the antiviral activity was contributed by cichoric acid, which we have previously shown to have moderate activity against HSV (Binns et al., Citation2002a), and possibly other caffeic acid derivatives present in these fractions. Nevertheless, there was no clear correlation between phenolic concentrations and relative activity, which suggests the presence of additional, perhaps more potent, antiviral compounds. In addition, some antiviral fractions were devoid of the marker phenolic compounds, thus reinforcing the concept that compounds other than caffeic acid derivatives were responsible for some of the activity. It is also feasible that several phenolic compounds could be acting synergistically, a possibility that could be tested by using combinations of the different pure compounds or by carrying out bioassay-guided fractionation.
The flower extract also contained water-soluble activity that was not due to photosensitizers and therefore could represent the same antiviral ingredients as the other aerial parts. These active fractions were also very rich in phenolic compounds, which probably at least contributed to some of this activity. In contrast, the ethanol extract had only marginal activity and therefore was missing the potent ethanol- and ethyl acetate-soluble activity found in leaves and stem extracts.
The water-soluble extracts would be expected to contain substantial polysaccharides, and such preparations have been shown previously to possess bioactivities, including some antiviral activity (Bauer, Citation1998). In the current study, the polysaccharide-rich extract (SG34) contained significant activity against HSV and FV (but not against RV), which was however relatively low in potency compared with the other aqueous fractions (they were all tested simultaneously and repeated several times). On this basis, it would seem unlikely that the polysaccharide/glycoprotein complex could be responsible for more than a small fraction of the water-soluble antiviral activity. In fact, the purified fractions derived from this extract (SG35, SG36) were found to have no detectable antiviral activity in our test system.
We conclude from these studies that the aerial parts of E. purpurea. contain multiple antiviral compounds, including more than one water-soluble compound, and at least one caffeic acid derivative, plus at least one ethyl acetate–soluble component that was a photosensitizer. This latter component was missing in the flower extract. These activities could between them contribute to the potent anti-HSV and anti-FV properties but could not explain the purported anti-rhinovirus efficacy of E. purpurea., as all the fractions were devoid of direct anti-RV activity. In addition, this overall profile of E. purpurea. antiviral activities was markedly different from the profiles shown by root preparations derived from several Echinacea. species (accompanying manuscript, this issue).
These results indicate that specific antiviral activities are present in our Echinacea. extracts, which are representative of commercial tinctures, teas, capsules, and so forth, and these may contribute to the purported benefits of Echinacea. preparations.
References
- Baron S, Singh I, Chopra A, Coppenhaver D, Pan J (2000): Innate antiviral defenses in body fluids and tissues. Antiv Res 48: 71–89. [CSA]
- Barrett B (2003): Medicinal properties of Echinacea.: A critical review. Phytomed 10: 66–86. [CSA], [CROSSREF]
- Bauer R (1998): Echinacea.: Biological effects and active principals. Phytomedicines of Europe: Chemistry and biological activity. In: Lawson LD, Bauer R, eds., ACS Symposium Series 691. Washington, DC, American Chemical Society, pp. 140–157.
- Bauer R, Khan A, Wagner H (1988): TLC and HPLC analysis of Echinacea pallida. and E. angustifolia. roots. Planta Med 54: 426–430. [CSA]
- Binns SE, Hudson J, Merali S, Arnason JT (2002a): Antiviral activity of characterized extracts from Echinacea. spp. (Heliantheae: Asteraceae) against herpes simplex virus (HSV-1). Planta Med 68: 780–783. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Binns S, Baum BR, Arnason JT (2002b): A taxonomic revision of the genus Echinacea. (Heliantheae: Asteraceae). Syst Bot 27: 610–632. [CSA]
- Goel V, Chang C, Slama JV, Barton R, Bauer R, Gahler R., Basu, TK (2002): Echinacea. stimulates macrophage function in the lung and spleen of normal rats. J Nutr Biochem 13: 487–492. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hudson JB, Towers GHN (1999): Phytomedicines as antivirals. Drugs of the Future 24: 295–320. [CSA], [CROSSREF]
- Merali S, Binns S, Paulin-Levasseur M, Ficker C, Smith M, Baum BR, Brovelli E, Arnason JT (2003): Antifungal and anti-inflammatory activity of the genus Echinacea.. Pharm Biol 41: 412–420. [CSA]
- Randolph RK, Gellenbeck K, Stonebrook K, Brovelli E, Qian Y, Bankaitis-Davis D, Cheronis J (2003): Regulation of human gene expression as influenced by a commercial blended Echinacea. product: Preliminary studies. Exp Biol Med 228: 1051–1056. [CSA]
- Rininger JA, Kickner S, Chigurupati P, McLean A, Franck Z (2000): Immunopharmacological activity of Echinacea. preparations following simulated digestion on murine macrophages and human peripheral blood cells. J Leuk Biol 68: 503–510. [CSA]
- Towers GHN, Page JE, Hudson JB (1997): Light-mediated biological activities of natural products from plants and fungi. Curr Org Chem 1: 395–414. [CSA]
