Abstract
Organic extracts of five medicinal plants (Alternanthera repens. (L) Kuntze, Boerhavia coccinea. Mill, Flaveria trinervia. (Spreng) C. Mohr, Tournefortia densiflora. M. et G., and Vitex mollis. Kunth), widely used in traditional Mexican medicine for the treatment of diarrhea and dysentery, were evaluated for antibacterial and intestinal antispasmodic activity. The antibacterial properties were determined in vitro. by the agar dilution method against multi-resistant enteric bacteria obtained from clinical isolates of Escherichia coli. R166 and R170, Salmonella typhi. R1234 and R 1330, and Enterobacter cloacae. R819, and against ATCC strains Shigella sonnei. 11060, Escherichia coli. 25922, Proteus mirabilis. 43071, and Salmonella typhimurium. 14028. In order to evaluate the effect of the extracts on the contraction induced in vitro., preparations of intestine from guinea-pigs were used. The integral extracts of B. coccinea. and T. densiflora. were the most active species with a MIC of <2 mg/ml. The highest antispasmodic activity was obtained with V. mollis. extract, 37% motility inhibition. These findings could be useful in the search for new therapeutic agents.
Introduction
In Mexico, diarrhea caused by bacterial and protozoal infections is one of the most common forms of gastrointestinal upset. This disease is associated with abdominal pain provoked by intestinal spasm. Failure in the treatment of this disease is due to bacterial resistance to therapeutic drugs. This is why studies on new natural compounds with antibacterial, antiprotozoal, or antispasmodic activity are necessary. Natural products are proposed as a therapeutic alternative to conventional antimicrobial treatment, whose effectiveness is often limited by the resistance that the infectious agents have developed against antibiotics (Ali et al., Citation1999; Nimri et al., Citation1999). Plants produce a great variety of compounds that originate from their secondary metabolism and accumulate in different parts of the plant. Recently, publications reporting the activity of medicinal plants against pathogenic microorganisms with multiple resistances to third- and fourth-generation antibiotics have appeared (Greenwood, Citation1998; Struelens, Citation1998). The herbaceous plants Alternanthera repens. (L) Kuntze, Boerhavia coccinea. Mill, Flaveria trinervia. (Spreng) C. Mohr, Tournefortia densiflora. M. et G., and Vitex mollis. Kunth grow wild in different areas of Mexico (Argüeta et al., Citation1994) and are used in traditional medicine for the treatment of diarrhea and dysentery (Tapia-Perez, 1999). These plants were selected to evaluate their biological properties as antibacterial and antispasmodic agents.
As a result of this investigation, data are made available for the first time concerning the antibacterial properties of whole extracts from these five plants against multiresistant enteric clinical isolates and on their antispasmodic effect in intestine from guinea-pigs.
Materials and Methods
Plant material
Leaves and stems from the plants were collected during 2001 in the state of Morelos, Mexico. Samples from each species were authenticated by Abigail Aguilar and deposited for identification and safe-keeping in the IMSSM Herbarium with the following registration numbers: Alternanthera repens. (L.) Kuntze (Amaranthaceae) (12368), Boerhavia coccinea. Mill (Nyctaginiaceae) (12276), Flaveria trinervia. (Spreng) C. Mohr (Asteraceae) (12255), Tournefortia densiflora. M. et G. (Boraginaceae) (12271), and Vitex mollis. Kunth (Verbenaceae) (13320).
Preparation of plant extracts
Leaves and stems were selected for the preparation of the extracts based on the ethnobotanical information (Tapia-Perez, 1999). Fresh biomass (1 kg) was dried in the dark at room temperature, and then leaves and stems were ground separately. The plants were exhaustively extracted by shaking with hexane or methanol for 48 h at room temperature. Extracts were dried under reduced pressure at 40°C, and the yield of solids obtained with respect to the starting dried biomass was determined. The samples were solubilized in 10% (v/v) dimethyl sulfoxide (DMSO) just before the biological assays were performed.
Microorganisms
Bacterial strains included in this work were ATCC strains Shigela sonnei. (11060), Escherichia coli. (25922), Proteus mirabilis. (43071), and Salmonella typhimurium. (14028). The five multiresistance enterobacterial clinical isolates were obtained from the bacterial collection of INSP/CISEI in Cuernavaca Morelos, Mexico, and were identified as Escherichia coli. (strains R166 and R170), Salmonella typhi. (strains R1234 and R 1330), and Enterobacter cloacae. (strain R819).
Susceptibility testing
Agar plates of Mueller-Hinton (MH) medium were prepared containing different concentrations (0, 2, 4, 8, and 16 mg/ml) of each of the five extracts following the recommendations of the National Committee for Clinical Laboratory Standards (NCCLS, Citation2002). Control plates including cefotaxime at 0, 2, 4, 8, and 16 µg/ml were included. Tests were done in duplicate and results expressed in mg/ml as a minimal inhibitory concentration (MIC) (Tilton & Howard, Citation1987); cefotaxime results are reported in µg/ml.
Spasmolytic assay
Induced contractions on the extracted intestine of guinea-pigs were used with the plant extracts according to the method established by Aguilar-Santamaria and Tortoriello (Citation1996). Male guinea-pigs weighing between 300 and 500 g were maintained at 22 ± 2°C, with a 12-h photoperiod with free access to food and water. Before the experiment, animals were fasted for 12 h and sacrificed by neck breaking. Ten to 12 cm of the smooth intestine (ileum) was removed and washed with Tyrode solution (mM): NaCl 136.9, NaHCO3 11.9, CaCl2 1.4, KCl 2.7, MgCl2 0.5, NaH2PO4 0.4, glucose 11.1; pH 7.4 at 37°C. Once washed, the tissue was maintained in Tyrode solution at 37°C and bubbled with O2 (95%) and CO2 (5%). A 3-cm tissue fragment was mounted in the intraluminal sprinkling chamber. Once the contractile activity was registered by a transformer connected to the Mac Lab equipment for recording data, the preparation was kept in repose for 30 min to stabilize. Below-threshold electric shocks were applied with a pair of electrodes using a Philipps and Bird stimulator 611. The stimuli were produced for a period of 5 s every 3 min with a frequency of 1 Hz during 10 ms and with an intensity of 20 V. Once the uniform reaction of the tissue had been recorded, the control register of the contraction (100%) was taken, and then varying concentrations of the plant extracts were applied (0.1, 1, 10, and 100 mg/ml). After 5 min, the tissues were once again stimulated, and the results obtained were compared with those of the control register. Assays were done in triplicate, and the results are reported according to the percentage of inhibition and were analyzed by the variant analysis (ANOVA) and the Student's t.-test.
Results and Discussion
The ethnobotanical characteristics of the five medicinal plants included in this study are shown in . Different parts of the plants are used in Mexican traditional medicine as antidiarrheal agents. Minimum inhibitory concentrations of the five medicinal plant extracts against four antibiotic-susceptible ATCC bacteria and the five multiresistance clinical isolates are shown in . In general, the hexane extracts of B. coccinea. (stems), A. repens. (aerial parts), and the methanol extracts of the stems of T. densiflora. were the most effective, as they had the lowest MIC values (< 2, 4, and 8 mg/ml, respectively, against the 9 bacteria tested). Specifically, the hexane extract of B. coccinea. stems and the methanol extract of T. densiflora. stems were active against Escherichia coli. R166 and R170 and Enterobacter cloacae. R819 clinical isolates. The hexane extract of B. cocinea. stems was also active against the S. typhi. R1330 and R1324 clinical isolates, with MIC values of 8 mg/ml. According to previous studies, the seeds of B. cocinea. have antimicrobial activity (Olukoya et al., Citation1993), although these authors did not report their effectiveness against multiresistant strains. In addition, results of antibiotic-sensitive bacteria (ATCC) versus multiresistance enterobacteria clinical isolates are very similar. These results suggest that multidrug resistant bacteria are susceptible to these extracts and they could be effective in treating gastric diseases caused by this group of bacteria.
Table 1. Ethnobotanical characteristics of the Mexican medicinal plants used in this study.
Table 2. Antibacterial activity of medicinal plant extractions against ATCC and multiresistance clinical isolates.
Leaves of V. mollis. and aerial parts of F. trinervia. were not active against the nine strains assayed (MIC > 16 mg/ml). Values for cefotaxime susceptibility correlates with the expected pattern; ATCC strains were susceptible (< 2 µg/ml) and clinical isolates were resistant (> 16 mg/ml) to this antibiotic.
It is important to point out that antibacterial compounds were found in whole extracts, which must be fractionated in order to isolate those compounds responsible for antimicrobial activity. It is reasonable to assume that one or several components of the extract have important antimicrobial activity. Further studies will be needed to identify the active compounds from the extracts and elucidate their mechanism of action on bacterial cells.
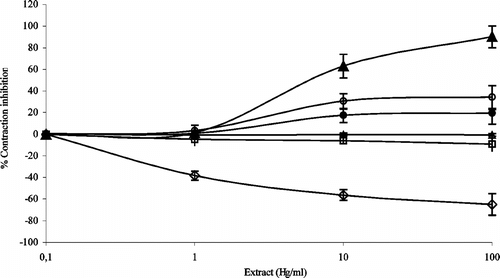
Extracts of the four plants showing the greatest antimicrobial activity [T. densiflora, B. coccinea.), and F. trinervia. and V. mollis. (Tapia-Pérez et al., Citation2003)] were selected to evaluate their effect on electrically induced contraction in guinea-pig intestine (). The hexane extracts of the aerial parts of T. densiflora. stimulated the contraction of the tissue in a concentration-dependent manner, whereas those from the tips of the T. densiflora. leaves and the aerial parts of F. trinervia. did not provoke any response. The hexane extract of B. coccinea. stems inhibited the electrically induced contraction by 12% at a concentration of 10 mg/ml in comparison with the control (papaverin). The V. mollis. plant extract had the greatest antispasmodic activity, inhibiting the contraction by 37% in a concentration-dependent manner. Previous pharmacological studies have shown that extracts of A. repens. (Zavala et al., Citation1980; Garcia et al., Citation1995) and the root of B. coccinea. (Ramabhimaiah et al., Citation1984) have antidiarrheal activity and stimulate smooth muscle contraction, respectively. The current work included extracts from T. densiflora., F. trinervia., and V. mollis. for which antispasmotic activity has not been reported previously.
Figure 1 Effect of the extracts of four medicinal plants on the electrically induced contraction using the example of extracted guinea pig's intestine. Each date is the mean and SD of five observation. Hexane extract of Tournefortia densiflora. leaves (⋄__⋄), hexane extract of Tournefortia densiflora. stem (•__•), methanol extract of Boerhavia coccinea. (•__•), extract of Flaveria trinervia. (+__+), methanol extract Tournefortia densiflora. leaves (▵___▵), methanol extract of Vitex mollis. (•__•), and the control papaverin (•___•).
B. coccinea. and A. repens. produce ursolic and oleonolic acid, whose antimicrobial activity has recently been confirmed (Woldemichael et al., Citation1999; Chattopadhyay et al., Citation2002).
The majority of the extracts tested in this study were active against the nine strains of bacteria, and those from two plants (B. coccinea. and T. densiflora.) had important antiprotozoal activity, as reported previously (Tapia-Perez et al., 2003). The ethnobotanical strategy was successful for the selection and evaluation of medicinal plants. In order to permit a clear identification of the compound with antibacterial and or antispasmodic activity, further studies will focus on the fractionation and isolation of the agent(s) responsible for the biological activity.
Acknowledgments
This work was supported by grant no. 30938 from CONACYT and FOFOI no. 2002-323-0003. Maria Esther Tapia Pérez was a M.Sc. from UAEM, Facultad de Medicina. The authors want to thank Professor Javier Palazón (Barcelona University) and Michael Dunn (Centro de Ciencias Genómicas, UNAM), Cuernavaca, Mor. Mexico, for reviewing the manuscript.
References
- Aguilar-Santamaría L, Tortoriello J (1996): Anticonvulsant and sedative effects of crude extracts of Ternstroemia pringlei. and Ruta chalepensis.. Phytother Res 10: 531–533. [CSA]
- Ali MS, Azhar I, Amtul Z, Ahmad VU, Usmanghani K (1999): Antimicrobial screening of some Caesalpiniaceae. Fitoterapia 70: 299–304. [CSA], [CROSSREF]
- Argüeta VA, Cano AL, Rodarte ME (1994): Atlas de las Plantas de la Medicina Tradicional Mexicana. Tomo I; II y III. Instituto Nacional Indigenista, pp. 78–680.
- Chattopadhyay D, Arunachalam G, Mandal AB, Sur TK, Mandal SC, Bhattacharya SK (2002): Antimicrobial and anti-inflammatory activity of folklore: Mallotus peltatus. leaf extract. J Ethnopharmacol 82: 229–237. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- García SB, Calderón CP, Fuentes LB (1995): Preliminary evaluation of the gastrointestinal activity of Alternanthera pungens.. Fitoterapia 66: 324–327. [CSA]
- Greenwood D (1998): Resistance to antimicrobial agents: A personal view. J Med Microbiol 47: 751–755. [PUBMED], [INFOTRIEVE], [CSA]
- NCCLS (National Committee for Clinical Laboratory Standards) (2002): Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Approved standard M100-S12. Wayne., PA, NCCLS.
- Nimri LF, Meqdam MM, Alkofahi A (1999): Antibacterial activity of Jordanian medicinal plants. Pharm Biol 37: 196–201. [CSA]
- Olukoya DK, Idika N, Odugbemi T (1993): Antibacterial activity of some medicinal plants from Nigeria. J. Ethnopharmacol 39: 69–72. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ramabhimaiah S, García IH, Pérez JK (1984): Pharmacological investigations on the water soluble fraction of methanol extract of Boerhavia diffusa. root. Indian Drugs 21: 343–344. [CSA]
- Struelens JM (1998): Tracking the epidemiology of antimicrobial drug resistance in hospitals: Time to deploy molecular typing. J Med Microbiol 47: 1035–1036. [PUBMED], [INFOTRIEVE], [CSA]
- Tapia-Pérez ME (1999): Plantas Medicinales Utilizadas para el Tratamiento de Padecimientos Gastrointestinales Infecciosos. Tesis de Licenciatura. Cuernavaca., Morelos., México, Facultad de Ciencias Biológicas, UAEM, pp. 1–170.
- Tapia-Pérez ME, Tapia-Contreras A, Cedillo-Rivera R, Osuna L, Meckes M (2003): Screening of mexican medicinal plants from antiprotozoal activity. Part II. Pharmaceutical Biol 41: 182–185. [CSA]
- Tilton RC, Howard BJ (1987): Antimicrobial susceptibility testing. In: Carson D, Bircher S, eds., Clinical and Pathogenic Microbiology. St. Louis, Mosby, pp. 121–156.
- Woldemichael GM, Singh MP, Maiese WM, Timmermann BN (1999): Constituents of antibacterial extract of Caesalpinia paraguariensis. Burk. Z Naturforsch 58: 70–75. [CSA]
- Zavala MA, Pérez GS, Hernández ZE, Pérez GRM, Pérez GC (1980): Antidiarrhoeic activity of different extracts of Alternanthera repens.. J Nat Prod 2: 60–67. [CSA]
