Abstract
Sida cordifolia. Linn. (Malvaceae) is commonly known as bala. and widely used in Ayurveda. The comparative antioxidant potential of ethanol extracts of Sida cordifolia. leaf, stem, root, and whole plant was studied. Anti–lipid peroxidation, free-radical scavenging, reducing power, nitric oxide scavenging, superoxide scavenging antioxidant assay, and further estimation of total phenolic content and HPTLC studies were carried out. Various antioxidant activities were compared with standard antioxidants such as BHA, α-tocopherol, and ascorbic acid. Ethanol extracts were found to be a good scavenger of DPPH radical in the order roots > stem > leaves > whole plant with values 76.62%, 63.87%, 58% and 29% at a dose of 1 mg, respectively. All extracts of Sida cordifolia. (SC) have effective reducing power and free-radical scavenging activity. Only the root extract exhibited superoxide-scavenging activity and inhibited lipid peroxidation in rat liver homogenate. All these antioxidant properties were concentration dependent. In addition, total phenolics content of all the extracts of S. cordifolia. were determined as gallic acid equivalents. The highest antioxidant activity was observed in the root extract. The results obtained in the current study indicate that S. cordifolia. is a potential source of natural antioxidants.
Introduction
Sida cordifolia. Linn (Malvaceae) is found in the Indian system of medicine (Ayurveda) and is known as bala.. The renowned Ayurvedic physician Charaka has categorized bala. as brmhaniya. (a bulk-promoting herb) and as balya. (tonic) and prajasthapana. (promotes reproduction). Charaka has also mentioned bala as rejuvenative (rasayana.) to muscle tissue and the muscular system (Paranjape, Citation2001). An aqueous extract of whole plant shows hepatoprotective activity against carbon tetrachloride, paracetamol, and rifampicin-induced hepatotoxicity (Kotoky & Das, 2000). Sitoindoside X, an acylsterylglycoside, from the roots of Sida cordifolia. has been proved as an adaptogenic and immunostimulant (Ghosal et al., Citation1988), while fumaric acid isolated from the plant showed hepatoprotective activity. The activity was compared to that of silymarin (Rao & Mishra, Citation1997). Reactive oxygen species (ROS) have various damaging effects. They initiate the peroxidation of the membrane lipids, leading to the accumulation of lipid peroxides (Halliwell & Gutteridge, Citation1989). The peroxidation products by themselves and their secondary oxidation products, such as malondialdehyde and 4-hydroxinonenal, are highly reactive. They react with biological substrates such as protein, amines, and DNA. Recent investigations have shown that the antioxidant property of various plants could be correlated with oxidative stress defense and different human diseases including cancer, atherosclerosis, aging, inflammation, and certain nervous system disorders (like Alzheimer disease). Antioxidants can interfere with the oxidation process by reacting with free radicals, chelating free catalytic metals, and also by acting as oxygen scavengers (Sanchez, Citation2002). Considering the traditional uses of this Ayurvedic drug, the preliminary phytochemical studies, and total polyphenolic determination, it was predicted to have antioxidant activity. Hence, the aim of this study was to investigate the antioxidant profile of different morphological organs of S. cordifolia. against free radicals using specific in vitro. models.
Materials and Methods
Chemicals
Chemicals used in this study were 1,1-diphenyl-2-picrylhydrazyl (DPPH) (Sigma no. D 9132), potassium ferricyanide, sodium nitrite, trichloroacetic acid, folin Ciocalteu reagent, sodium nitroprusside, sulfanilamide, N.-1-naphthylethylenediamine dihydrochloride, butylated hydroxy anisole (BHA), butylated hydroxy toluene (BHT), ascorbic acid (Merck [India] cat. no. 61809501001046), thiobarbituric acid, tocopheryl acetate. All reagents used for the experiments were of analytical grade.
Collection of plant materials
Plant material
Sida cordifolia. was collected from surrounding local areas on 5 September 2003 (autumn season). The plant was authenticated by the Botanical Survey of India (Pune, India). A voucher specimen (no. 689) was also deposited there. The plant was cleaned and dried in the shade, then powdered to 40 mesh and stored in an airtight container at 25°C.
Extraction
Sida cordifolia. roots, stems, leaves, and whole plant (100 g each) in powdered form were extracted with ethanol (90%), by cold maceration for 48 h and then filtered, and solvent was evaporated under reduced pressure in rotary evaporator, and we obtained 2.5 g (leaves), 3.8 g (stem), 2.7 g (root), and 1 g (whole plant) as dried extracts.
Phytochemical evaluation
The stock solution was prepared by dissolving 500 mg of ethanol extract in 20 ml of methanol, and each was subjected to preliminary phytochemical testing for the detection of major chemical groups (Kokate et al., Citation1990).
Thin-layer chromatography (TLC) fingerprint profile of Sida cordifolia.
TLC fingerprint profile of ethanol extract of roots, leaves and stems and whole plant was established using HPTLC Ethanol extract solution (10 µg/µL) was spotted on a precoated Silica-gel 60 F254 TLC using Camag Linomat IV automatic sample spotter, and the plate was developed in the solvent system of chloroform: glacial acetic acid:methanol:water (6:3.2:1.2:0.8). The plate was dried at room temperature and scanned using Camag TLC Scanner 3 at UV 254 nm, and Rf values and peak area of resolved bands were recorded. The TLC was derivatized by vanillin–sulfuric acid and natural product–polyethylene glycol (NP/PEG) agent for the detection of phenolic compounds, especially flavonoids (Wagner & Bladt, Citation1996).
Estimation of total phenolics
The total phenolic contents of ethanol extracts were determined with Folin-Ciocalteu reagent according to Slinard & Singleton (Citation1977) and slightly modified. The stock solution of extract 1 mg/ml in water was prepared. From the stock solution, 5 ml of solution was transferred to a 25-ml volumetric flask, the volume was made up with distilled water, and out of this sample 5 ml or standard 2 ml was taken in 25-ml volumetric flask, to this 10 ml of distilled water, and 2 ml of phenol reagent (20%) was added, and then the volume was made up with 29% sodium bicarbonate. The mixture was kept in dark for 20 min, after which the absorbance at 760 nm was noted. Standard used was gallic acid, and the total polyphenolic content was calculated as gallic acid, equivalents and expressed in % as gallic acid.
Antioxidant assay
Free-radical scavenging activity
The free-radical scavenging activities of S. cordifolia. extracts were measured by decrease in the absorbance of methanol solution of DPPH. A stock solution of DPPH (33 mg in 1 l) was prepared in methanol, which gave initial absorbance of 0.8, and 5 ml of this stock solution was added to 1 ml of S. cordifolia. extract solution at different concentrations (250–2500 µg). After 30 min, absorbance was measured at 517 nm. Antiradical activity was calculated as % inhibition from the given formula:
Reducing power assay
The reducing power of S. cordifolia. was determined as per the reported method (Oyaizu, Citation1986). Different concentrations of S. cordifolia. extracts (250–2500 µg) in 1 ml of ethanol were mixed with phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferrocyanide (2.5 ml, 1%). The mixture was incubated at 50°C for 20 min. A portion (2.5 ml) of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. The upper layer of the solution (2.5 ml) was mixed with distilled water (2.5 ml) and FeCl3 (0.5 ml, 0.1%) and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power.
Nitric oxide scavenging activity
The procedure is based on the principle that sodium nitroprusside solution at physiological pH spontaneously generates nitric oxide, which interacts with oxygen to produce nitrite ions, which can be estimated using Griess reagent. Scavenger of nitric oxide competes with oxygen, leading to reduced the production of nitrite ions. The assay was performed according to method described by Sreejayan and Rao (Citation1997).
Different concentrations of the extract sample were prepared in 100 ml, to which 0.1489 g of sodium nitroprusside (final conc. 5 mM) was added and incubated at room temperature. At different time intervals, 5.6 ml of reaction mixture was taken out and 0.2 ml of Griess reagent A (1% sulfanilamide in 5% phosphoric acid) was added, and kept at 30°C for 10 min. After incubation, 0.2 ml of Griess reagent B (0.1% N.-1-naphthylethylenediamine dihydrochloride) was added and kept at 30°C for 20 min. After incubation, absorbance was measured at 542 nm against blank. The same reaction without the extract sample but equivalent amount of ethanol served as control. Concentration of NO· was calculated from standard calibration curve of sodium nitrite solution of varying concentration.
Superoxide radical scavenging assay
The assay was based on the method described by Nishikimi et al. (Citation1972), and this was slightly modified. The reaction mixture consisting of 1 ml of nitro blue tetrazolium (NBT) solution (156 µM NBT in phosphate buffer, pH 7.4), 1 ml NADH solution (468 µM NADH in phosphate buffer, pH 7.4), and 1 ml of sample solution of SC extracts was mixed. The reaction were started by adding 100 µl of phenazine methosulfate (PMS) solution (60 µM PMS in phosphate buffer, pH 7.4) to the mixture. The reaction mixture was incubated at 25°C for 5 min and the absorbance at 560 nm was measured against blank sample. Decreased absorbance of reaction mixture indicated increased superoxide anion scavenging activity.
Anti–lipid peroxidation assay in rat liver homogenate
Lipid peroxidation in rat liver homogenate was evaluated by the TBA method (Ohkawa et al., Citation1979; Sreejayan & Rao, Citation1994). The reaction mixture containing 0.5 ml (10%) rat liver homogenate, 1 ml of 0.15 M KCl, and 0.5 ml of different concentrations of drug extract were prepared. Lipid peroxidation was initiated by adding 100 µl of 1 mM ferric chloride solution. The reaction mixtures were incubated for 30 min at 37°C. After incubation, the reaction was stopped by adding 2 ml ice-cold thiobarbituric acid solution containing 15% trichloroacetic acid, 0.38% thiobarbituric acid in 0.25 M HCl and 0.05% butylated hydroxy toluene. The reaction mixtures were heated for 60 min at 80°C, cooled, and centrifuged at 6900 rpm for 15 min. The absorbance of supernatant was measured at 532 nm against a blank, which contained all reagents except liver homogenate and drug. Identical experiments were performed to determine the normal (without drug and FeCl3) and induced (without drug). The percentage of anti-lipid peroxidation effect (ALP%) was calculated by following formula:
For the experimentation of anti–lipid peroxidation assay of rat liver homogenate, the work was conducted in accordance with standard institutional guidelines, with a prior approval of the animal experimentation committee.
The values of all the above experiments were represented as the average of experiments repeated on three different days.
Results
From preliminary phytochemical evaluation and HPTLC fingerprinting of ethanol extracts of S. cordifolia., it was confirmed that major groups were alkaloids (using Mayer's reagent and Dragendorff's reagent) and flavonoids (using Shinoda's test). By derivatizing with natural product–PEG reagent, all the extracts showed green fluorescence in varying amount, and that of the root extract was intense as compared with other extracts.
The intensity of the test positive for the compound was represented in an arbitrary scoring of 1 to 5, where five represent the maximum amount of the compounds present ().
Table 1.. Phytochemical analysis of Sida cordifolia.
The free radical scavenging activity was demonstrated by DPPH and nitric oxide. DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule (Blois, Citation1958). DPPH radical was usually used as a substrate to evaluate antioxidant activity of antioxidants. It involves reaction of specific antioxidant with a stable free radical 2,2-diphenyl-1-picryl-hydrazyl DPPH* (C18H12N506 M = 394.33). As a result, there is reduction of DPPH concentration by antioxidant, which decreases the optical absorbance of DPPH; this is detected by spectrophotometer at 517 nm.
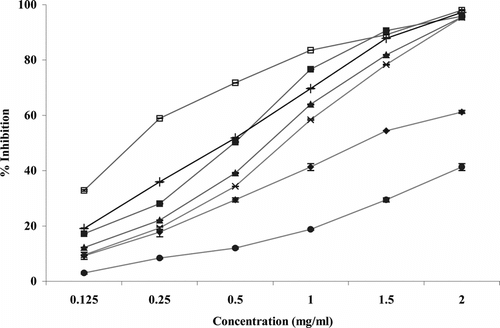
illustrates a significant decrease in the concentration of DPPH radical due to the scavenging ability of all extracts of S. cordifolia.. BHA and tocopherol were used as standards. The scavenging effect of different extracts on the DPPH radical decreased in the order root > stem > leaf > whole plant and were 76.62%, 63.87%, 58% and 29%, respectively, at a concentration of 1000 µg/ml. These results indicated that all extracts have a noticeable effect on scavenging the free radicals. Free-radical scavenging activity of all the extracts were concentration dependent.
Figure 1 Free-radical scavenging activity of Sida cordifolia. extracts, BHA, ascorbic acid, and α.-tocopherol by 1,1-diphenyl-2-picrylhydrazyl radicals. Results are mean ± SD of three parallel readings. (♦ whole plant, ▪ roots, ▴ stem, × leaves, + ascorbic acid, • α- tocopherol, □ BHA.)
The nitric oxide scavenging property of the extracts showed negative results or could not be assessed, as the color components of the extracts may be interfering with the measurement of the chromophore formed in the reaction mixture.
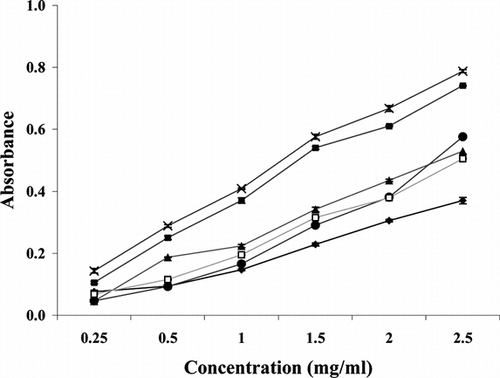
For the measurements of the reducing ability, “Fe3+–Fe2+ transformation” in the presence of all the extracts of S. cordifolia. was found (Oyaizu, Citation1986). The reductive capabilities of extracts of S. cordifolia. were compared with tocopherol and BHA. The reducing power of SC was found to increase with rising concentrations (). All of the extracts of SC showed less activity as compared with that of BHA.
Figure 2 Reducing power of Sida cordifolia. ethanol extracts, BHA, and α.-tocopherol. Results are mean ± SD of three parallel measurements. Spectrophotometric detection of the Fe+3–Fe+2 transformation. (♦ whole plant, × roots, ▴ stem, ▪ leaves, • α-tocopherol, □ BHA.)
In the PMS-NADH-NBT system, superoxide anion derived from dissolved oxygen, by PMS-NADH coupling reaction, reduces NBT (yellow dye) to blue-colored product called formazan. Drugs possessing superoxide scavenging activity decreases the reduction of NBT, which is a measure of superoxide anion scavenging activity that is indicated by reduction in absorbance at 560 nm. In the superoxide scavenging assay, only root extract has shown decrease in absorbance against increase in concentration in the concentration range 250–2500 µg in . IC50 values for BHT were found to be 20.15 µg ± 0.63 and for root extract 630.5 µg ± 0.33. Other extracts did not show scavenging activity; it may be because of interference of color.
Figure 3 Superoxide anion radical scavenging activity of S. cordifolia. roots extracts and BHT by PMS-NADH-NBT method. Results are mean ± SD of three parallel measurements.
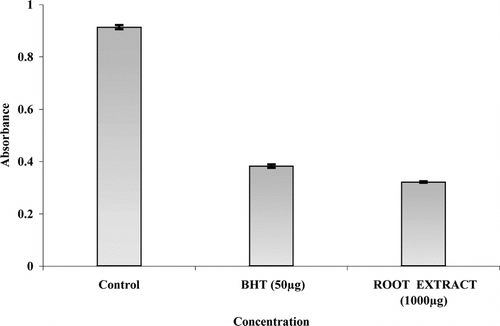
Figure 4 Inhibitory effect of Sida cordifolia. roots extract at different doses on FeCl3-induced lipid peroxidation in rat liver homogenate. Results are mean ± SD of three parallel measurements.
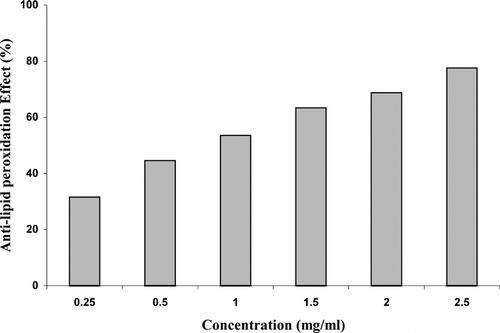
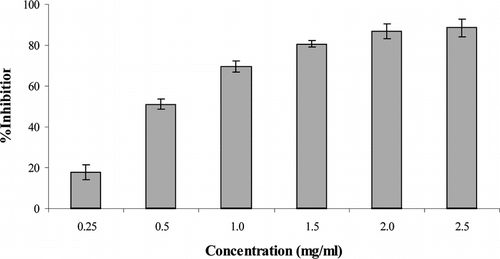
The anti–lipid peroxidation effect of root extract was observed in FeCl3-induced lipid peroxidation in rat liver and brain homogenate against control. Anti–lipid peroxidation effect of S. cordifolia. roots ethanol extract is shown in and . A concentration range 0.25–2.5 mg/ml in liver homogenate showed 31.55–77.55% inhibition, whereas in brain homogenate it showed 16.0–96.86%. These results show that inhibition of TBARS formation in rat liver and brain homogenate increased by increasing concentrations.
Figure 5 Inhibitory effect of Sida cordifolia. roots extract at different doses on FeCl3-induced lipid peroxidation in rat brain homogenate. Results are mean ± SD of three parallel measurements.
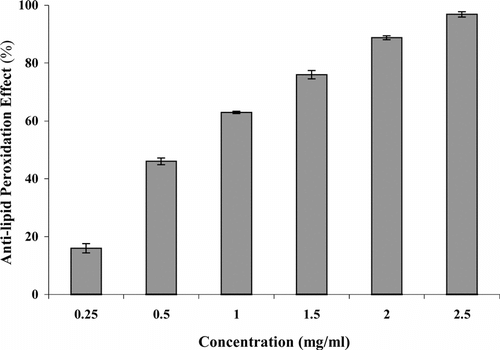
Figure 6 Inhibitory effect of Sida cordifolia. root extract at different doses on FeCl3-induced lipid peroxidation in rat brain homogenate. Results are mean ± SD of three parallel measurements.
From the results of total polyphenolic content, it was found out that there were 1.6%, 2.2%, 2.9%, and 4.1% of gallic acid equivalents of phenolic compound in 100 mg of the whole plant, stem, leaves, and root extracts of S. cordifolia., respectively. The results indicate that there is a correlation between antioxidant activity and total phenolic content ().
Table 2.. Antioxidant profile of Sida cordifolia.
Discussion
Sida cordifolia. is one of the widely used drugs in various Ayurvedic and herbal formulations. Certain plants show antioxidant activity because of their phenolic constituents. Flavonoids are a broad class of low-molecular-weight, secondary metabolites widely distributed in plants. The beneficial effects of flavonoids are attributed to their antioxidant and chelating abilities (Heim et al., Citation2002). Phytochemical analysis and the HPTLC fingerprint confirmed the presence of phenolics, especially flavonoids. From the results, the root extract has shown significant antioxidant property as compared with whole plant, leaf, and stem extracts. The antioxidant activity of roots may be attributed to its flavonoid content. S. cordifolia. extracts have shown reducing capacity. Studies have indicated that the antioxidant effect is related to development of reductones. Reductones are reported to be terminators of free-radical chain reactions (Dorman et al., Citation2003); thus the antioxidant activity of S. cordifolia. extracts may be related to its reductive activity. Prevention of lipid peroxidation in rat liver/brain homogenate confirmed that it is active against effects of free radicals on biological membranes. It has also shown superoxide radical scavenging activity.
The only other member from the same family Malvaceae that shows activity against superoxide and lipid peroxidation is gossypin of Gossypium herbaceum. (Babu et al., Citation2003). Other plants showing activity against superoxide radical are Hypericum perforatum. (Tripathi & Pandey, Citation1999), Garcinia indica. (Yagamachi et al., Citation2000), Green tea (Nakagawa & Yokozawa, Citation2002), Hemidescus indicus. (Mary et al., Citation2003), volatile oil of seeds of Nigella sativa. L. (Osama et al., Citation2003), Moringa oleifera. (Siddhuraju & Becker, Citation2003), and seeds of Foeniculum vulgare. (Oktay et al., Citation2003). This literature survey undertaken by the authors also indicates that only one plant from the same family and very few other plants have such type of activity. The activity of S.cordifolia. against superoxide radical is of significance because superoxide can decrease the activity of other antioxidant defense enzymes such as catalase and glutathione peroxidase as well as being cytotoxic by generating more reactive species like peroxy nitrite (Halliwell & Gurreridge, Citation1989). Superoxide radicals are more detrimental due to their role as second messengers in fibroblast proliferation in inflammation and mediators of tissue destruction (Windrow et al., Citation1993).
It is known that cleavage products of lipid peroxidation accumulate in the central nervous system and in cardiac and muscle fibers (Nohl, Citation1993). S. cordifolia. has been found to reduce lipid peroxidation. This may be considered as a corroboration of Ayurvedic claims for the plant. Ayurveda has recommended roots, and indeed through this current project all parts including whole plant have also been evaluated, indicating that the roots have more pronounced antioxidant effects.
References
- Babu BH, Jayram HN, Nair MG, Ajaikumar KB, Padikkala J (2003): Free radical scavenging, antitumor and anticarcinogenic activity of gossypin. J Exp Clin Cancer Res 22: 581–589. [PUBMED], [INFOTRIEVE], [CSA]
- Blois MS (1958): Antioxidant determination by the use of stable free radical. Nature 181: 1199–1200. [CSA]
- Dorman DHJ, Kosar M, Kahlos K, Holm Y, Hiltunen R (2003): Antioxidant properties and composition of aqueous extracts from Mentha. species, hybrids, varieties and cultivars. J Agric Food Chem 51: 563–569. [CSA], [CROSSREF]
- Ghosal S, Kaur R, Bhattacharya SK (1988): Chemistry and bioactivity of sitoindosides IX and X. Planta Med 54: 561. [CSA]
- Halliwell B, Gutteridge JMC (1989): Free Radicals in Biology and Medicine. Oxford, Oxford University Press, pp. 23, 130.
- Heim KE, Tagliaferro AR, Bobilya DJ (2002): Flavonoid antioxidants: Chemistry, metabolism and structure activity relationships. J Nutr Biochem 13: 572–584. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kokate CK, Purohit AP, Gokhale SB (1990): Pharmacognosy. Pune, India, Nirali Prakashan, pp. 106–108.
- Kotoky J, Das PN (2001): Hepatoprotective activity of Sida cordifolia. roots against carbon tetrachloride intoxicated rats. J Med Arom Plant Sci 23: 100–107. [CSA]
- Mary NK, Achuthan CR, Babu BH, Padikkala J (2003): In vitro antioxidant activity and anti-thrombotic activity of Hemidescus indicus.. J Ethnopharmacol 87: 187–191. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Nakagawa T, Yokozawa T (2002): Direct scavenging of nitric oxide and superoxide by Green tea. Food Chem Toxicol 40: 1745–1750. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Nishikimi M, Rao NA, Yagi K (1972): The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46: 849–853. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Nohl H (1993): Involvement of free radicals in ageing: A consequence or cause of senescence. Br Med Bull 49: 653–667. [PUBMED], [INFOTRIEVE], [CSA]
- Ohkawa H, Ohishi N, Yagi K (1979): Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem 95: 351–358. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Oktay M, Gülcin I, Küfrevioglu ÖI (2003): Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare.) seed extracts. Lebensm Wiss Technol 36: 263–271. [CSA], [CROSSREF]
- Osama A, Badary RA, Taha AM, Gamal El-Din Mohamed H, Abdel-Wahab (2003): Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol 26: 87–98. [CSA], [CROSSREF]
- Oyaizu M (1986): Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 307–315. [CSA]
- Paranjape P (2001): Indian Medicinal Plants: Forgotten Healer: A Guide to Ayurvedic Herbal Medicine. Delhi, Chaukhamba Sanskrit pratisthan, p. 39.
- Rao KS, Mishra SH (1997): Isolation and assessment of hepatoprotective activity of fumaric acid obtained for the first time from Sida cordifolia.. Indian Drugs 34: 702–706. [CSA]
- Sanchez CM (2002): Review: Methods used to evaluate the free radical scavenging activity in foods and biological systems. J Food Sci Tech Int 8: 121–137. [CSA], [CROSSREF]
- Siddhuraju P, Becker K (2003): Antioxidant properties of various solvent extracts of total phenolic constituents from three different agro climatic origins of the drumstick tree (Moringa oleifera. Lam.) leaves. J Agri Food Chem 51: 2144– 2155. [CSA], [CROSSREF]
- Slinard K, Singleton VL (1977): Total phenol analysis automation and comparison with manual methods. Am J Enol Vitic 49–55. [CSA]
- Sreejayan, Rao MNA (1994): Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol 46: 1013–1016. [PUBMED], [INFOTRIEVE], [CSA]
- Sreejayan, Rao MNA (1997): Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol 49: 105–107. [PUBMED], [INFOTRIEVE], [CSA]
- Tripathi YB, Pandey E (1999): Role of alcoholic extract of shoot of Hypericum perforatum. Linn. on lipid peroxidation and various species of free radicals in rats. Indian J Exp Biol 37: 567–571. [PUBMED], [INFOTRIEVE], [CSA]
- Wanger H, Bladt S (1996): Plant Drug Analysis: A Thin Layer Chromatography Atlas. Berlin, Springer-Verlag, p. 197.
- Windrow VR, Winyard PG, Morris CJ, Blake DR (1993): Free radicals in inflammation: Second messengers and mediators of tissue destruction. Br Med Bull 49: 506–522. [CSA]
- Yagamachi F, Saito M, Ariga T, Yoshimura Y, Nakazawa H (2000): Antioxidative and anti-glycation activity of garcinol from Garcinia indica. fruit rind. J Agri Food Chem 48: 180–185. [CSA], [CROSSREF]
