Abstract
Leaves of Piper betle. Linn (Piperaceae) possess a broad spectrum of pharmacological and therapeutic properties. However, its antinociceptive activity has not been investigated so far. The aim of this study therefore, was to examine the antinociceptive activity of hot water extract (HWE) and cold ethanol extract (CEE) of P. betle. leaves using rats and three models of nociception (tail flick, hot plate, and formalin tests). Different concentrations of HWE (125, 200, 300, 500 mg/kg) and CEE (125, 200, 300, 500 mg/kg) were made and orally administrated to rats, and the reaction times were determined. The results showed that the extracts have marked antinociceptive activity when evaluated in the hot plate and the formalin tests but not in the tail-flick test. The overall antinociceptive effect of CEE was higher than that of HWE. The antinociceptive effect was mediated via opioid mechanisms.
Introduction
Piper betle. Linn (Piperaceae) is a perennial dioecious, semi-woody climber. Stems strongly swollen at the nodes, papillose when young. Leaves alternate, simple, and yellowish green to bright-green in color. Leaves of fertile branches with a petiole 1–2 cm long, 1.2–1.8 mm thick when dry, and glabrous at maturity. Flowers are naked, unisexual, dioecious in dense cylindrical spikes, male spikes not seen, female spikes 2.5–5 cm long, pendulous. Bracts peltate, orbicular to obcordate, broadly stipitate with a membranous margin (Jayaweera, Citation1982; Dassanayake & Fosberg, Citation1987). P. betle. is cultivated in Sri Lanka, India, Malay Peninsula, the Philippines, and East Africa (Dassanayake & Fosberg, Citation1987). The chief constituent of the leaves of this plant is a volatile oil known as betel oil. The volatile oil is a bright-yellow to dark-brown liquid possessing a clove-like flavor and consists of terpenes and phenols (Anonymous, Citation1992).
In Asian countries, betel leaves are used for chewing and are credited with many medicinal properties such as digestive, stimulative, carminative, and aphrodisiac (Anonymous, Citation1992). However, Sri Lankan betel inhibits male sexual behavior in rats and possesses antiaphrodisiac activity (Ratnasooriya & Premakumara, Citation1996). Further, betel juice is given to children for cough and administered to the eye for night blindness in adults. It is used to treat catarrh and diphtheria. The leaves are given for gastric and lung disorders in children and applied to purulent ulcers (Jayaweera, Citation1982). Experimentally, leaves of P. betle. are shown to possess antimicrobial (Tewari & Nayak, Citation1991), gastroprotective (Majumdar et al., Citation2003), wound healing (Santhanam & Nagarajan, 2002), hepatoprotective (Saravanan et al., Citation2002), antioxidant (Choudhary & Kale, Citation2002; Saravanan et al., Citation2002; Santhakumari et al., Citation2003), antifertility on male rats (Ratnasooriya & Premakumara, Citation1997), and antimotility effects on washed human spermatozoa (Ratnasooriya et al., Citation1990). According to available literature, antinociceptive activity of P. betle. is not scientifically investigated yet. However, it is possible that P. betle. leaves may possess antinociceptive properties, as P. longum. (Vedhanayaki et al., Citation2003) a close relative of the plant, was shown to have antinociceptive properties. Therefore, this study was undertaken to examine whether extracts of leaves of P. betle. possess antinociceptive activity. This was tested in rats using oral administration of hot water and cold ethanol extracts.
Materials and Methods
Plant material
P. betle. leaves were purchased from the main vegetable markets in the western province of Sri Lanka in May 2002. The leaves were identified and authenticated by the curator of National Herbarium, Royal Botanical Gardens, Peradeniya, Sri Lanka. A voucher specimen (PS 01) was deposited in the Industrial Technology Institute, Colombo, Sri Lanka.
Animals
Healthy adult cross-bred male albino rats (weighing 200–250 g) were used throughout the experiment. They were housed under standard environmental conditions with free access to pelleted food (Vet House Ltd., Colombo, Sri Lanka) and tap water.
Preparation of hot water extract (HWE)
P. betle. leaves were air-dried for 3–5 days in the shade and cut into small pieces. Five hundred grams were boiled with 2.5 l of distilled water (DW) for 4 h. The hot water extract was concentrated under vacuum (yield 26.2% w/w, dry weight basis) and stored at 4°C until use.
Preparation of cold ethanol extract (CEE)
P. betle. leaves were air dried for 3–5 days in the shade and cut into small pieces. Five hundred grams were macerated with ethanol (80% v/v) and kept for 48 h at room temperature (28–30°C). The extraction was filtered and the filtrate was evaporated to dryness under reduced pressure (yield 15.6% w/w, dry weight basis) and stored at 4°C until use.
Administration of extracts
Doses of 125, 200, 300, and 500 mg/kg of HWE and CEE were prepared in 1 ml of DW and given orally to separate groups (n = 12 or 6 per group per extract) of rats. Doses selected were comparable to what has been generally used in investigating pharmacological activities of herbal extracts (Saravanan et al., Citation2002; Majumdar et al., Citation2003).
Phytochemical screening of hwe and CEE
Extracts were subjected to qualitative testing for alkaloids, polyphenols, steroids, saponins, and tannins as described by Farnsworth (Citation1996).
Evaluation of antinociceptive activity
Hot-plate and tail-flick tests
The reaction times of rats were measured 1 h prior to the treatment (pretreatment) and then at hourly intervals for 5 h after the treatment (post-treatment) either with HWE, CEE, vehicle, or reference drug using hot-plate and tail-flick techniques as described by Langerman et al. (Citation1995). In the hot-plate test, the rat was placed on an enclosed hot plate (Model MK 35 A, Muromachi Kikai Co. Ltd., Tokyo, Japan) maintained at 50°C and the time taken (in seconds) either to lick the hind paw or jump from the surface of the hot plate (the reaction time) was determined. In the tail-flick test, the tail of the rat was immersed (5–6 cm from the tip) in a water bath at 55°C and the time taken (in seconds) to flick the tail was determined by using a stop watch. Rats showing a pretreatment reaction time greater than 15 s in the hot-plate test and 5 s in the tail-flick test were not used in the experiment. A cutoff time of 25 s was set to avoid tissue damage. The reference drug, pethidine (25 mg/kg), was injected intramuscularly to a separate group (n = 9 per group) of rats.
Formalin test
The method of Dubuission and Dennis (Citation1977) was used with slight modifications to evaluate the antinociceptive activity. In brief, 1 h after the administration (either of CEE, HWE, vehicle, or reference drug), each rat was injected subcutaneously with 50 µl of 2.5% formalin solution into the subplantar surface of the left hind paw. Rats were then observed for 60 min, and the time spent licking the injected paw was recorded in two phases. The first phase, 1–5 min post–formalin injection, is known as the early phase and the period between 15–60 min as the late phase. The reference drug, aspirin (100 mg/kg), was given orally to another group of rats (n = 9 per group).
Mechanism for antinociceptive activity
This was investigated using CEE because the antinociceptive activity was higher compared to HWE. Further, 200 mg/kg was selected because the maximal antinociceptieve activity was evident with this dose.
Investigation of involvement of opioid receptor
Eighteen rats were randomly divided into two equal groups. Rats in group 1 were injected subcutaneously with 5 mg/kg of naloxone hydrochloride in 1 ml of normal saline. For group 2, 1 ml of normal saline was injected subcutaneously. After 45 min, both groups of rats were given 200 mg/kg of CEE orally, kept for 1 h, and examined on the hot plate.
Investigation of involvement of dopamine receptor
Eighteen rats were randomly divided into two equal groups. Rats in group 1 were orally administered 1.5 mg/kg of metoclopramide, a dopamine (D2) antagonist, in 1 ml of 1% methyl cellulose. Rats in group 2 were orally treated with 1 ml of 1% methyl cellulose. After 1 h, both groups of rats were orally treated with 200 mg/kg of CEE extract and the hot-plate test performed.
Sedative effect of the extract
Eighteen rats were randomly divided into two equal groups. One group was treated with 1 ml of distilled water (DW) and the other group was treated with 200 mg/kg of CEE in 1 ml of DW. After 1 h, each rat was placed in the center of the rat hole-board and observed for 7.5 min. The number of rears, number of head dips, locomotor activity, and number of fecal boluses were recorded as described by File and Wardill (Citation1975).
Effect of the extract on muscle relaxation and muscular coordination
Eighteen rats were randomly divided into two equal groups. Group 1 was treated with 200 mg/kg of CEE in 1 ml of DW and group 2 was treated with 1 ml of DW. After 1 h, these rats were subjected to the bar-holding test (to evaluate the muscle relaxation) followed by the bridge test (to evaluate the muscle coordination), and the latency to fall and slide off (in seconds) were determined, respectively as described by Plaznic et al. (Citation1993).
Statistical analysis
Data are given as means ± SEM. Statistical comparisons were made using one-way ANOVA followed by Tukey's family error test. A p value ≤0.05 was considered as significant. ID50 values were determined graphically.
Results
Phytochemical screening of HWE and CEE
Phytochemical screening revealed the presence of alkaloids, polyphenols, steroids, saponins, and tanins in both extracts.
Hot-plate and tail-flick tests
In the hot-plate test, significant (p < 0.05) prolongation of the reaction time was evident with all the tested doses of CEE () at 2 h post-treatment. Further prolongation of the reaction time lasted up to 5 h (until the termination of the experiment) with 200 and 300 mg/kg doses. On the other hand, only 200 and 300 mg/kg doses of HWE significantly (p < 0.05) prolonged the reaction time up to 5 h and 3 h, respectively (). The ID50 for the increase in the reaction time at 1 h after the treatment of HWE and CEE was 145 mg/kg and 92 mg/kg, respectively. The best antinociceptive activity was evident with 200 mg/kg dose of both CEE and HWE. Further antinociceptive effect of 200 mg/kg dose of CEE was comparable to the reference drug pethidine at each point of time (CEE: 1 h, 105%; 2 h, 77%; 3 h, 76%; 4 h, 56%; 5 h, 35%; pethidine: 1 h, 118%; 2 h, 73%; 3 h, 61%; 4 h, 44%; 5 h, 41%). Likewise, the antinociceptive effect of 200 mg/kg dose of HWE also was comparable to the pethidine at each point of time apart from the 1 h (HWE: 1 h, 84%; 2 h, 61%; 3 h, 67%; 4 h, 45%; 5 h, 32%).
Figure 1 Effect of different doses of cold ethanol extract (CEE) of P. betle. leaves on the reaction time of rats (n = 9; hot-plate test, means ± SEM). *p < 0.05 as compared with control.
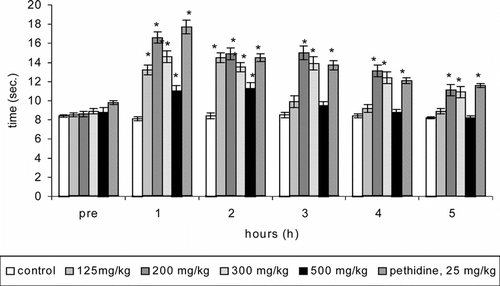
Figure 2 Effect of different doses of hot water extract (HWE) of P. betle. leaves on the reaction time of rats (n = 9; hot-plate test, means ± SEM). *p < 0.05 as compared with control.
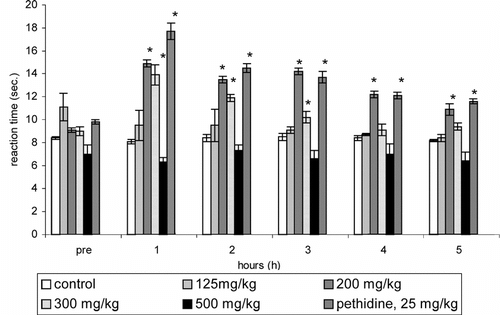
There was no significant increase in the reaction time with any of the doses of HWE or CEE in the tail-flick test (data not shown).
Formalin test
All the tested doses of CEE significantly (p < 0.05) reduced the licking time of the formalin-injected paw in the early phase (by 17–52%). However, other tested doses of CEE showed a significant (p < 0.05) reduction of the licking time in the late phase (by 12–22%) apart from the 125 and 500 mg/kg doses. On the other hand, only 200 and 300 mg/kg doses of HWE significantly (p < 0.05) reduced the licking time in both the early (by 17–32%) and late (by 13–17%) phases. Compared to the control, the reference drug, aspirin, significantly (p < 0.05) reduced the licking time in both early (by 65%) and late (by 75%) phases ().
Table 1. Effect of different doses of hot water extract (HWE) and cold ethanol extract (CEE) of P. betle. leaves on the reaction time of rats (formalin test, means ± SEM).
Investigation of involvement of opioid receptor
In the naloxone study, with the hot-plate technique, subcutaneous administration of naloxone significantly (p = 0.0025) impaired the reaction time induced by 200 mg/kg dose of CEE ().
Table 2.. Effect of naloxone on the reaction time of rats induced by 200 mg/kg dose of cold ethanol extract (CEE) of P. betle. leaves (hot-plate test, means ± SEM).
Investigation of involvement of dopamine receptor
In the metoclopramide experiment, with the hot-plate technique, the oral administration of metoclopramide did not significantly (p > 0.05) change the reaction time induced by the 200 mg/kg dose of CEE ().
Table 3.. Effect of metoclopramide on the reaction time of rats induced by 200 mg/kg dose of cold ethanol extract (CEE) of P. betle. leaves (hot-plate test, means ± SEM).
Sedative effect of the extract
In the rat hole-board tests, none of the parameters investigated was significantly (p > 0.05) altered by the dose 200 mg/kg of CEE (control vs. treatment: number of rears 28.9 ± 1.0 vs. 29.8 ± 0.9, number of head dips 13.7 ± 0.5 vs. 14.2 ± 0.9, number of crossings 17.1 ± 0.6 vs. 15.1 ± 0.8, and number of fecal boluses 0.77 ± 0.34 vs. 0.88 ± 0.33).
Effect of the extract on muscle relaxation and muscular coordination
When compared with control, 200 mg/kg dose of CEE did not significantly (p > 0.05) change the latency to fall in the bar-holding test (control vs. treatment 58.9 ± 1.0 vs. 59.2 ± 0.9 s) or the latency to slide off in the bridge test (control vs. treatment 58.1 ± 0.4 vs. 58.3 ± 1.7 s).
Discussion
The results demonstrate that extracts of P. betle. leaves have antinociceptive activity as evaluated in the hot-plate test and not in the tail-flick test. This indicates centrally mediated antinociceptive activity of the plant extracts against the acute pain: both hot-plate and tail-flick tests measure centrally medicated transient pain. (Lopez–Munoz et al., Citation1993). The hot-plate test predominately measures supraspinally organized responses, wherein the tail-flick test predominately measures spinal reflexes (Wong et al., Citation1994). Because both extracts are effective only against hot-plate test, the antinociceptive is likely to mediated supraspinally. The lack of activity in the tail-flick method could be due to coexistence of compound(s) that interacts with other receptors such as α.2-adrenoceptors in the spinal cord (Ossipov et al., Citation1990). The antinociceptive activity of extracts was genuine as there was no change in the retain times of bar and bridge tests as compared with controls. The highest antinociceptive activity was evident with 200 mg/kg dose of both HWE and CEE. Further, antinociceptive activity of CEE was higher than that of HWE extract in terms of ID50 values. The dose-response curves of the P. betle. extracts were bell-shaped. Such an action may result from desensitization (Seuka & Mazrzymas, Citation1991) or downregulation of receptors (Stewart & Badiani, Citation1993). Bell-shaped dose-response curves have been reported with other synthetic (Li et al., Citation1996) and herbal (Arambewela et al., Citation2004) analgesics.
Both 200 and 300 mg/kg doses of P. betle. extracts markedly reduced the licking time in early and late phases of the formalin test in a bell-shaped dose-response curve. In the formalin test, the pain in the early phase is caused due to the direct stimulation of the sensory nerve fibers by formalin, whereas the pain in the late phase is due to the inflammatory mediators, like histamine, prostaglandin, serotonin, and bradykinin (Murray et al., Citation1988; Tjolsen et al., Citation1992). It is reported that NSAIDs reduce both phases of the formalin test (Martindale et al., Citation2001). Therefore, extracts induced interruptions of both phases of this test, suggesting possible impairments of sensory transmission and release of inflammatory mediators.
Some sedatives possess antinociceptive activity (Rang et al., Citation1995). However, the antinociceptive action of CEE is unlikely to be mediated via sedation as none of the parameters monitored in the rat hole-board technique was changed. Antinociception can be induced via dopaminergic mechanisms (Jensen & Yaksh, Citation1986), but such a mode of action is unlikely in this study as metoclopramide, a dopamine receptor antagonist, failed to block antinociception induced by the extract. The opioid receptor, antagonist naloxone, blocked the antinociception induced by the CEE, suggesting that the antinociception was mediated through opioid mechanisms. Further alkaloids were present in the CEE, and alkaloids, which possess antinociceptive properties usually, act via opioid mechanisms (Badio et al., Citation1995). The overall antinociceptive effect of CEE was higher than that of HWE. In CEE, most of chemical compounds kept intact without major changes. In hot water extract, there is a strong possibility that some of the heat-labile constituents are destroyed. According to Arambewela et al. (Citation2003), both extracts were devoid of unacceptable side effects even after chronic administration at a dose of 1500 mg/kg. There were no overt signs of toxicity, hepatotoxicity (in terms of serum AST, ALT levels), or renotoxicity (as judged by serum urea and creatine levels).
In conclusion, our results demonstrate, for the first time, antinociceptive activity of P. betle. leaves, and further studies will be undertaken to correlate the antinociceptive activity with the chemical constituents. It may be possible to develop safe and potent antinociceptive agents from P. betle. leaves.
Acknowledgments
The authors express their gratitude to Mr. J.R.A.C. Jayakody, University of Colombo, Department of Zoology, for assisting in this study and National Science Foundation for the research grant (SIDA (1L) 2000/BT/03).
References
- Anonymous (1992): The Wealth of India. The Dictionary of Indian Raw Materials and Industrial Products. Raw Material Vol. 5, Publication and Information directorate, CSIR, pp. 84–94.
- Arambewela LSR, Arawwawala LDAM, Ratnasooriya WD (2004): Antinociceptive activities of aqueous and etahnolic extracts of Alpinia calcarata. rhizomes in rats. J Ethnopharmacol 95: 311–316. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Arambewela LSR, Arawwawala LDAM, Ratnasooriya WD (2003): Safety evaluation of Sri Lankan Piper betle. leaf extracts in rats. J Trop Med Plants 4: 195–198. [CSA]
- Badio B, Shi D, Garraffo HM, Daily JN (1995): Antinociceptive effects of the alkaloid epibatidine: Further studies on involvement of nicotine receptors. Drug Develop Res 36: 46–59. [CSA], [CROSSREF]
- Choudhary D, Kale RK (2002): Antioxidant and nontoxic properties of Piper betle. leaf extract: in vitro. and in vivo. studies. Phytother Res 61: 461–466. [CSA], [CROSSREF]
- Dassanayake MD, Fosberg FR (1987): A Revised Hand Book to the Flora of Ceylon. New Delhi, Amreind, pp. 287–288.
- Dubuission D, Dennis SG (1977): The formalin test: A quantitative study of the analgesia effects of morphine, meperidine and brain stem stimulation in rats and cats. Pain 4: 167–174. [CSA]
- Farnsworth NR (1996): Biological and phytochemical screening of plants. J Pharm Sci 55: 225–276. [CSA]
- File SE, Wardill A (1975): Validity of head dipping as a measure of exploration in modified hole-board. Psychopharmacol 44: 53–57. [CSA], [CROSSREF]
- Jayaweera DMA (1982): Medicinal Plants Used in Ceylon, Vol. 5. Colombo, National Science Council of Sri Lanka, p. 201.
- Jensen TS, Yaksh TL (1986): Effects of an intrathecal dopamine agonists, apomorphine, on thermal and chemical evoked noxious responses in rats. Brain Res 296: 285–293. [CSA], [CROSSREF]
- Langerman L, Zakouski ML, Piskoun B, Grant GJ (1995): Hot plate versus tail flick: Evaluation of acute tolerance to continuous morphine infusion in the rat model. J Pharmacol Toxicol Methods 34: 23–28. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Li BY, Nalwalk JW, Barker LA, Cumming P, Parsons MF, Hougn LB (1996): Characterization of the antinociceptive properties of cimetidine and a structural analog. J Pharmacol Exp Ther 276: 500–508. [PUBMED], [INFOTRIEVE], [CSA]
- Lopez–Munoz FJ, Salazar LA, Castaneda–Hernandes G, Villarreal JE (1993): A new model to assess analgesic activity: Pain induced functional impairement in the rat (PIFIR). Drug Develop Res 28: 169–175. [CSA], [CROSSREF]
- Majumdar B, Chaudhuri SGR, Ray A, Bandyopadhyay SK (2003): Effect of ethanol extract of Piper betle. Linn leaf on healing of NSAID-induced experimental ulcer—A novel role of free radical scavenging action. Indian J Exp Biol 41: 311–315. [PUBMED], [INFOTRIEVE], [CSA]
- Martindale J, Bland–Ward PA, Chessell IP (2001): Inhibition of C-fibre mediated sensory transmission in the rat following intraplantar formalin. Neurosci Lett 316: 33–36. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Murray CW, Porreca F, Cowan A (1988): Methodological refinements in the mouse paw formalin test an animal model of tonic pain. J Pharmacol Methods 20: 175–186. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ossipov MH, Harris S, Lloyd P, Messineo E, Lin BS, Bagley J (1990): Antinociceptive interaction between opioids and medetomidine: Systemic additivity and spinal synergy. Anesthesiology 73: 1227–1235. [PUBMED], [INFOTRIEVE], [CSA]
- Plaznic A, Stefanski R, Palejko W, Kotawski W (1993): The role of accumbense GABA-B receptors in the regulation of rat behavior. Neurosci Res Common 12: 23–30. [CSA]
- Rang HP, Dale MM, Ritter JM (1995): Pharmacology. London, Churchill Livingstone.
- Ratnasooriya WD, Premakumara GAS (1996): Piper betle. leaves impairs masculine sexual behavior of rats. Med Sci Res 24: 303–306. [CSA]
- Ratnasooriya WD, Premakumara GAS (1997): Piper betle. leaves reversibly inhibits fertility of male rats. Vidyodaya J Sci 7: 15–21. [CSA]
- Ratnasooriya WD, Jayawardena KGI, Premakumara GAS (1990): Antimotility effects of Piper betle. (L) leaf extract on washed human spermatozoa. J Natl Sci Coun Sri Lanka 18: 53–60. [CSA]
- Santhanam G, Nagarajan S (1990): Wound healing activity of Curcuma aromatica. and Piper betle.. Fitoterapia 61: 458–459. [CSA]
- Saravanan R, Prakasam A, Ramesh B, Pugalendi KV (2002): Influence of Piper betle. on hepatic marker enzymes and tissue antioxidant status in ethanol-treated wistar rats. J Med Food 5: 197–204. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Santhakumari P, Prakasam A, Pugalendi KV (2003): Modulation of oxidative stress parameters by treatment with Piper betle. leaf in streptozotocin induced diabetic rats. Indian J Pharmacol 35: 373–378. [CSA]
- Scuka M, Mazrzymas JW (1991): Post synaptic potentiation and desensitization at the vertebrate end-plate receptors. Prog Neurobio 38: 19–33. [CSA], [CROSSREF]
- Stewart J, Badiani A (1993): Tolerance and sensitization to the behavioural effects of drugs. Behavi Pharmacol 4: 289–312. [CSA]
- Tewari SN, Nayak M (1991): Activity of four plant leaf extracts against three fungal pathogens of rice. Trop Agric 68: 373–375. [CSA]
- Tjolsen A, Berge DG, Hunskaar S, Rosland JH, Hole K (1992): The formalin test: An evaluation of the method. Pain 51: 5–17. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Vedhanayaki G, Shastri GV, Kuruvilla A (2003): Analgesic activity of Piper longum. Linn. root. Indian J Exp Biol 41: 649–651. [PUBMED], [INFOTRIEVE], [CSA]
- Wong CH, Day P, Yarmush J, Wu W, Zbuzek UK (1994): Nifedipine-induced analgesia after epidural injections in rats. Anesthes Analges 79: 303–306. [CSA]
