Abstract
The current study was performed to evaluate the effect of the methanol extract of Cissus quadrangularis. (L.) (CQE) against free-radical damage. The test extract exhibited significant scavenging effect on DPPH free radical, superoxide radical, hydroxyl radical production, and inhibition of lipid peroxide production in erythrocytes. The free-radical scavenging effect of CQE was comparable with that of reference antioxidants. The activities of liver marker enzymes and antioxidant defense enzymes in rat liver homogenate were assessed in CCl4- and CQE-treated animals. Carbon tetrachloride (CCl4) caused a significant increase in aspartate aminotransferase (AST) and alanine aminotransferase (ALT), alkaline phosphatase (ALP), malondialdehyde (MDL) levels and a decrease in superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione-S.-transferase (GST), and reduced glutathione (GSH) activities, which were reverted by CQE treatment. The results obtained suggest that CQE showed antilipid peroxidative, free-radical scavenging property and ameliorated the liver damage by an increase in antioxidant enzymes activities. It can be concluded that the free-radical scavenging/antioxidant activity of the plant extract may be responsible for the therapeutic action against tissue damage.
Introduction
Oxygen-derived free radicals, such as the superoxide anion and hydroxyl radical, are cytotoxic and promote tissue injury (Peterhans, Citation1997). Oxidative stress, the consequence of an imbalance of pro-oxidants and antioxidants in the organism, is gaining recognition as a key phenomenon in chronic illness like inflammatory and heart diseases, hypertension, and gastrointestinal disorders (Oh et al., Citation2001). Antioxidants act as a major defense against radical-mediated toxicity by protecting the damage caused by free radicals. Furthermore although medicinal plants are used as “antioxidants” in traditional medicine, their claimed therapeutic properties could be due, in part, to their capacity for scavenging oxygen free-radicals (Nayan & Janardhanan, Citation2000).
Cissus quadrangularis. (L.) (Vitaceae) is commonly known as “bone setter”; the plant is referred to as asthisamdhani. in Sanskrit (Sivarajan & Balachandran, Citation1994). The stout fleshy quandrangular stem of Cissus quadrangularis. is an edible plant found throughout the hotter parts of India, Malaysia, West Africa, and Ceylon (Udupa et al., Citation1970). The plant is frequently used as a common food item in India. The plant is used medicinally in the indigenous systems of medicine both in the Ayurvedic and Unani systems. The stem is alterative, anthelmintic, dyspeptic, digestive, tonic, analgesic in eye and ear diseases, used for irregular menstruation, asthma, piles, tumors, fractures of bones, wounds, and scurvy (Kritikar & Basu, Citation2000).
The plant has been reported to contain high amount of dietary antioxidants that includes vitamin C, carotenoids, and polyphenols (Tiangburanatham, Citation1996; Chidambara Murthy et al., Citation2003). Other constituents include triterpene (α-amyrin and α-amyrone) (Bhutani et al., Citation1984), phytosterol (β-sitosterol) (Pleumaj & Saifah, Citation1986), ketosteroid (Udupa et al., Citation1965), oxo-steroid (Sen, Citation1964), two asymmetrical tetracyclic triterpenoids, namely onocer-7-ene-3α, 21β-diol and onocer-7-ene3β, 21α-diol (Madan & Ram, Citation1990), and calcium have been detected by phytochemical screening. Cissus quadrangularis. has been reported to possess analgesic, antiosteoporotic, antimicrobial, and antioxidant properties (Singh et al., Citation1984; Chidamabara Murthy et al., Citation2003; Shirwaikar et al., Citation2003).
We have previously reported that the methanol extract of Cissus quadrangularis. (CQE) produced antiulcer effect on indomethacin-induced gastrointestinal toxicity in rats (Mallika & Shyamala Devi, 2004). In view of the ethnobotanical uses of Cissus quadrangularis. as described above, the effect of CQE on lipid peroxidation in the erythrocytes, DPPH free radicals, superoxide anion, hydroxyl radical production, and CCl4-induced hepatic damage in rats were studied.
Materials and Methods
Plant material
The stem parts of Cissus quadrangularis. used in this study were purchased from Native Care and Cure Center (India). The plant was authenticated by Dr. P. Brindha, Pharmacognosy Department, Captain Srinivasa Murthy Drug Research Institute for Ayurveda, (Chennai, India). A voucher specimen has been deposited in the herbarium of the same institute.
Preparation of plant extract
Dried stem parts of Cissus quadrangularis. were coarsely powdered, and 1 kg of this powdered plant material was soaked in 2 l methanol for 48 h and the extract was filtered and distilled on a water bath. The last traces of the solvent were removed under vaccum drier, and the solid brown mass obtained was stored at − 4°C until further use. The yield of the extract was 5.2% w/w of powdered methanolic extract. For in vitro. antioxidant assay, the lyophilized extract was dissolved in 0.9% saline. For in vivo. assay, CQE was suspended in distilled water and administered orally to animals.
Phytochemical screening
Phytochemical studies (Kokate et al., Citation1996) on this extract revealed the presence of triterpenes including α- and β-amyrins, β-sitosterol, ketosteroids, phenols, saponins, tannins, carotene, and vitamin C. Quantitative estimation showed that Cissus quadrangularis. plant contains moisture 12.1; protein, 10.4; fat and wax, 1.0; fiber, 13.4; carbohydrates, 37.2; mucilage and pectins, 1.1; and ash, 16.7%.
In vitro. antioxidant assay
Lipid peroxidation in erythrocytes
Studies on erythrocyte lipid peroxidation in the presence or absence (control) of extract were carried out as described by De Azevedo et al. (Citation2000) with slight modifications. Human red blood cells obtained from healthy donors were washed three times in cold phosphate-buffered saline (PBS) by centrifugation at 3500 rpm. After the last washing, cells were suspended in PBS and its density adjusted to 1 mM hemoglobin in each reaction tube. The final cell suspensions were incubated with different concentrations of the test compounds dissolved in dimethyl sulfoxide (DMSO) and PBS during 10 min at 37°C. After incubation, cells were exposed to ter-butylhydroperoxide (1 mM) during 15 min at 37°C under vigorous shaking. After treatment with different concentration of extract (100–1000 µg/ml), lipid peroxidation was determined indirectly by thiobarbitoric acid reaction substance (TBARS) formation as described previously, and percentage of inhibition was calculated (De Azevedo et al., Citation2000). BHT is a well-known free-radical scavenger, which was used as a standard.
DPPH decoloration assay
The scavenging of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals was assayed following the method of Hantano et al. (Citation1989). The extract dissolved in methanol at different concentrations (100–1000 µg/ml of the final volume) was added with an equal volume of DPPH solution (0.1 mM) and was shaken vigorously. The mixture was incubated at room temperature for 50 min before the absorbance at 517 nm was read. Catechin was used as a reference free-radical scavenger. The scavenging activity was determined by comparing the absorbance with that of blank (100%) that containing only DPPH and solvent.
Superoxide anion
Superoxide radical (O2−) was generated from the photoreduction of riboflavin and was deducted by nitro blue tetrazolium dye (NBT) reduction method in the absence (control) and presence of the extract. The superoxide generated in this reaction sequence reduces the NBT, leading to a chromophore with a maximum of absorption at 560 nm. The activity was measured spectrophotometrically as reported previously (Paya et al., Citation1992; Masaki et al., Citation1995).
Hydroxyl radical scavenging activity assay
Hydroxyl radical scavenging potency of the test extract was assayed according to Klein and colleagues as described previously (Prashanth Kumar et al., Citation2000). Briefly, the reaction mixture contained EDTA (0.1 µM), Fe3+ (167 µM as a 1:2 mixture with EDTA), DMSO (33 µM) in 50 mM phosphate buffer. The extract was added at different concentrations (100–1000 µg/ml of the final volume) separately. The reaction was started by the addition of 2 mM ascorbic acid. The mixture was incubated at 37°C for 30 min and the reaction was stopped by the addition of 125 µl TCA. The formaldehyde formed during the oxidation of the DMSO by the Fe3+–ascorbic acid system was assayed spectrohotometrically, and the percentage of inhibition was calculated as described above.
In vivo. antioxidant assay
Animals
Male albino rats of weight 150–200 g were purchased from Tamil Nadu University of Veterinary and Animal Sciences (Chennai, India). The animals were housed at 27 ± 2°C in temperature, 55% in humidity, and a 12-h light/dark cycle. They were fed with standard laboratory chow (Hindustan Lever Foods, Bangalore, India) and provided with water ad libitum.. Experimental protocols were approved by our institutional ethical committee, which follows the guidelines of CPSCEA, which complies with international norms of INSA.
Acute toxicity
The acute toxicity study of CQE was studied on adult male albino rats at a dose of 0.5, 1.5, 3.0, and 5.0 g/kg body weight per day. Animals were watched carefully for 72 h after CQE administration and then for the next 7 days. At the end of this experimental period, the rats were observed for signs of toxicity, morphological behavior, and mortality (Mallika Jainu & Shyamala Devi, Citation2004).
Biochemical analysis
Rats were divided into 4 groups of 6 animals each. Group I: Normal rats were given the distilled water alone. Group II: Rats were administered carbon tetrachloride (CCl4) (1.25 ml/kg, p.o.) alone. Group III: Rats were pretreated with CQE orally at a dose 350 mg/kg for 7 days, and on the seventh day 30 min after the last dose, CCl4 p.o. was administered. Group IV: Rats administered with the same dose of CCl4 p.o. as mentioned above, and 30 min later CQE (350 mg/kg) was administered post-treatment orally for 7 days. Group V: Rats were pretreated with silymarin (100 mg/kg) for 7 days, and on the seventh day CCl4 was administered p.o. 30 min after the last dose of silymarin. Thirty-six hours after the experimental period, rats in all the groups were sacrificed, and liver tissues were dissected out for the estimation of aspartate aminotransferase (AST), and alanine aminotransferase (ALT) (Mohur & Cooke, Citation1975) and alkaline phosphatase (ALP) were also assayed by the method of King (Citation1965). The induction of malondialdehyde (MDL) formation by hydrogen peroxide, ascorbate, and ferrous sulfate was assayed according to Sumathi et al. (Citation1996). Superoxide dismutase (SOD) (Misra & Fridovich, Citation1972), catalase (CAT) (Sinha, Citation1972), glutathione peroxidase (GPx) (Rotruck et al., Citation1973), glutathione-S.-transferase (GST) (Habig et al., Citation1974), and reduced glutathione (GSH) (Moron et al., Citation1979) were also estimated. The dosage and duration of treatment for the above-mentioned groups have been fixed based on the effect of CQE on serum AST, ALT, ALP enzymes and bilirubin level (data not shown).
Statistical analysis
The values are expressed as mean ± SD. The results were computed statistically (SPSS software package, version 7.5) using one-way analysis of variance. Dunnett's T3 post hoc. testing was performed for inter-group comparison. p < 0.001, < 0.01, < 0.05 was considered significant.
Results
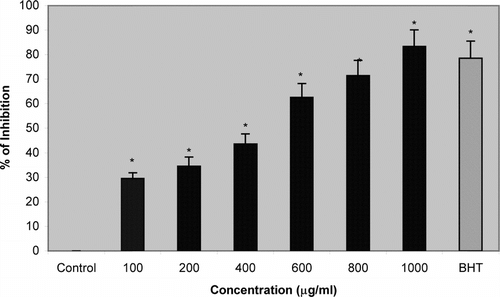
The results of the investigations revealed that CQE at doses of 100–1000 µg/ml inhibits lipid peroxidation in erythrocytes by 29.5%, 34.5%, 43.6%, 62.6%, 71.5%, and 83.4%, respectively whereas BHT, a standard antioxidant, inhibits lipid peroxidation by 78.6% as compared with control. CQE at a dose of 1000 µg/ml showed a significant antilipid peroxidative effect that is more potent than BHT ().
Figure 1 Effect of methanol extract of Cissus quadrangularis. (CQE) on lipid peroxide production in human erythrocytes. Data represents mean ± SD. *p < 0.001, statistically significant from control.
shows the role of CQE on DPPH model, superoxide anion, and hydroxyl radical formation. In the range of concentrations used, the test extract showed a dose-dependent scavenging activity on DPPH radical, superoxide anion, and hydroxyl radicals as compared with control. At higher dose of CQE (1000 µg/ml), the percentage of inhibition on DPPH radical and hydroxyl radicals were increased by 75.6% and 80.1%, and catechin showed an inhibition of 79.3% and 78.4%, respectively. From a comparison of the hydroxyl radical scavenging effect of CQE with catechin, it seemed that CQE was more effective than catechin. In vitro. superoxide scavenging action of the plant extract showed 75.3% inhibitory effect on superoxide anion, as compared with 79.9% inhibition by a standard antioxidant.
Table 1.. In vitro. antioxidant/free-radical scavenging activity of CQE on DPPH, superoxide anion, and hydroxyl free-radical formation
Acute toxicity studies revealed that CQE was found to be practically nontoxic when administered orally to rats, and its LD50 value was found to be higher than 5 g/kg. No lethality or any toxic reactions were found up to the end of the study period. Administration of CCl4 to rats caused significant liver damage, as evidenced by the altered serum biochemical parameters. shows the level of LPO in the liver tissues of control and experimental animals. In CCl4-induced rats (group II), there was a significant increase of MDL release under induced conditions (p < 0.001). Postreatment with CQE (group IV) resulted in a significant fall in the level of MDL (p < 0.05), whereas upon CQE pretreatment (group III), the levels of MDL were brought down to near normal levels in CCl4-induced rats, the results being comparable to those obtained with silymarin (group V).
Table 2.. Effect of CQE and silymarin on AST, ALT, and ALP enzymes in CCl4-induced rats
A significant increase (p < 0.001) in the liver marker enzymes and decrease (p < 0.001) in antioxidant enzyme activities were observed in CCl4-administered rats (group II) when compared with control. Post-treatment with CQE (group IV) significantly decreased (p < 0.05) the CCl4-induced alterations on AST, ALT, and ALP activities and increased (p < 0.05) the SOD, CAT, GPx, GST, and GSH levels when compared with group II rats. A marked decrease (p < 0.001) in AST, ALT, ALP activities and a significant increase (p < 0.001) in the liver antioxidant enzymes were observed in CQE-pretreated rats after the administration of CCl4 (group III). The hepatic protection against CCl4 damage in CQE post-treated rats was less than that of CQE pretreated rats. The CQE showed significant hepatoprotective activity against CCl4, comparable with the standard silymarin (Tables and ).
Table 3.. Effect of CQE and silymarin on the levels of tissue malondialdehyde in CCl4-induced rats
Table 4.. Effect of CQE and silymarin on SOD, CAT, GPx, GST enzymes and GSH levels in CCl4-induced rats
Discussion
Oxidative stress, the consequence of an imbalance of pro-oxidants and antioxidants in the organism, is gaining recognition as a key phenomenon in chronic illness like inflammatory and heart diseases, hypertension, and some forms of cancer (Oh et al., Citation2001). Plant-based, antioxidant-rich foods traditionally formed the major part of the human diet, and plant-based dietary antioxidants are hypothesized to have an important role in maintaining human health (Iris Benzie, Citation2003). In order to determine if CQE is capable of reducing oxidative stress, the production of TBARS in erythrocytes was assessed. In the range of concentrations used, CQE showed a dose-dependent inhibitory effect on the production of TBARS. Therefore, extract showed suppressive activities on lipid peroxidation in erythrocytes that may reveal its therapeutic potentials for several inflammatory diseases.
Another way of measuring antioxidant potential is by determining the free-radical inhibitory ability of CQE by using very stable free radicals such as DPPH. The principle of the reduction of DPPH free radical is that the antioxidant reacts with the stable free radical DPPH and converts it to 1,1-diphenyl-2-picryl hydrazine (Sreeyan & Rao, Citation1996). CQE showed an inhibition of free-radical production, which is nearly equipotent to catechin. In the current study, the scavenging effect of CQE in DPPH model is attributed to the hydrogen-donating ability of Cissus quadrangularis. (Shimada et al., Citation1992). Superoxide radical is a highly toxic species, which is generated by numerous biological and photochemical reactions (Govindarajan et al., Citation2003). The activity toward superoxide anion was mainly due to the presence of β-carotene in CQE (Chidambara Murthy et al., Citation2003) that scavenges superoxide radical and suppresses singlet oxygen. Inhibition of Fe3+ ascorbate mediated oxidative damage of deoxyribose was found to achieve up to 80.1% as compared with the inhibitory effect of catechin, a known hydroxyl scavenging agent. Nair et al. (Citation2000) have suggested that vitamin C, the major constituent of Cissus quadrangularis., has in vitro. lipid peroxidation inhibition capability and scavenging action on peroxyl and hydroxyl radicals (Ramanathan et al., Citation2003).
CCl4 administration to rats causes changes in liver marker enzymes and antioxidant enzymes, which is due to the damaged liver parenchymal cells (Singh, Citation1980). Administration of CCl4 elevated the levels of AST, ALT, and ALP significantly, due to its enzymatic activation of CCl3 free radical, which in turn alters the structure and function of liver cells (Singh et al., Citation1998). It has been emphasized that one of the important causes of CCl4-induced liver injury is LPO by free-radical derivatives of CCl4 (Venukumar & Latha, Citation2002). CQE were effective in reducing MDL formation by increase in CAT activity and GSH levels. The data obtained from our current study show that the extract pretreatment significantly decreases the liver marker enzymes, ameliorates the CCl4-induced changes in rats by increase in the antioxidant enzyme activities, and showed marked protection than post-treatment with CQE.
The phytochemical studies carried out on the extract revealed the presence of vitamin C, carotenoids, triterpenes, saponins, and phenolic substances, which have been known to be excellent antioxidants (Ramanathan et al., Citation2003; Wattarapenpaiboon & Wahlqvist, Citation2003). These antioxidative constituents present in CQE might be responsible for the free-radical scavenging and antioxidant activity. Supplementation of the antioxidant defense system of the organism with natural antioxidant compounds derived from plants may have beneficial effects because it has been shown that free-radical scavengers prevent hepatic damage (Raju et al., 2001). Thus the antioxidant activity and the scavenging action of free radicals by CQE might be important in the protection against CCl4-induced hepatopathy.
There is enough evidence to support that extract from Cissus quadrangularis. exhibited strong antioxidant activity and free-radical scavenging effect in different in vitro. and in vivo. systems. Furthermore, it may be suggested that the Cissus quadrangularis. extracts tested possess nontoxic, antioxidant activities, which renders them suitable as potential therapeutics, thus making them excellent candidates for more detailed investigation. Further work is necessary to isolate active principles and elucidate the actual mechanism involved in the antioxidant activity of this plant.
References
- Bhutani KK, Kapoor R, Atal CK (1984): Two unsymmetrical tetracylic triterpenoids from Cissus quadrangularis.. Phytochemistry 23: 407–410. [CSA]
- Chidambara Murthy KN, Vanitha A, Mahadeva Swamy M, Ravishankar GA (2003): Antioxidant and antimicrobial activity of Cissus quadranqularis. Linn. J Med Food 6: 99–105. [PUBMED], [CSA], [CROSSREF]
- De Azevedo MBM, Alderete J, Rodríguez JA, Souza AO, Rettori D, Torsoni MA, Faljoni-Alario A, Haun M, Duran N (2000): Biological activities of new antitumoral indole derivatives in an inclusion complex with cyclodextrin. J Incl Pheno Macro Chem 37: 93–101. [CSA], [CROSSREF]
- Govindarajan R, Vijaya kumar M, Rawat AKS, Shanta M (2003): Free radical scavenging potential of Picorrhiza kurroa Royle Ex Benth.. Ind J Exp Biol 41: 875–879. [CSA]
- Habig WH, Pabst MJ, Jackpoby WB (1974): Glutathione-S-transferase. A first enzymatic step in mercapturic acid formation. J Biol Chem 249: 7130–7139. [PUBMED], [INFOTRIEVE], [CSA]
- Hantano T, Edamatsu R, Mori A, Fujita Y, Yasuhara T, Yoshida T, Okuda T (1989): Phenolic constituents of licorice. II. Structures of licopyranocoumarin, licoarylcoumarin and glisoflavone, and inhibitory effects of licorice phenolics on xanthine oxidase. Chem Pharm Bull 37: 3005–3009. [CSA]
- Iris Benzie FF (2003): Evolution of dietary antioxidants. Comp Biochem Physiol A Mol Integ Physiol 136: 113–126. [CSA], [CROSSREF]
- Jainu M, Shyamala Devi CS (2004): Effect of Cissus quadrangularis. on gastric mucosal defensive factors in experimentally induced gastric ulcer – a comparative study with sucralfate. J Med Food 7: 372–376. [PUBMED], [INFOTRIEVE], [CSA]
- King J (1965): The hydrolases—acid and alkaline phosphatses. In: Practical Clinical Enzymology. London, D. Van Nostrand Co. Ltd., pp. 191–196.
- Kokate CK, Purohit CK, Gokhale SB (1996): Phytochemical tests. In: Pharmacognosy. Pune, India, Nirali Prakashan, pp. 510–512.
- Kritikar KR, Basu BD (2000): In: Basu LM, ed., Indian Medicinal Plants, Third revised and enlarged ed. pp 841–843.
- Madan MG, Ram KV (1990): Unsymmetric tetracylic triterpenoid from C. quadrangularis.. Phytochemistry 29: 336–337. [CSA], [CROSSREF]
- Masaki HS, Sakaki T, Atsumi T, Sakurai H (1995): Active oxygen scavenging activity of plant extracts. Biol Pharm Bull 18: 162–166. [PUBMED], [INFOTRIEVE], [CSA]
- Misra HP, Fridovich I (1972): The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247: 3170–3175. [PUBMED], [INFOTRIEVE], [CSA]
- Mohur AF, Cooke IJY (1975): Simple method of measuring serum level of glutamate oxaloacetic acid and glutamate pyruvate transaminase in routine laboratories. J Clin Pathol 10: 394–399. [CSA]
- Moron NS, Depierr JW, Mannervik B (1979): Levels of glutathione, glutathione reductase and glutathione-S-transferase activities in rat lung and liver. Biochem Biophys Acta 582: 67–72. [PUBMED], [INFOTRIEVE], [CSA]
- Nair S, Norkus EP, Hertan H, Pitchumoni CS (2000): Micronutrient antioxidants in gastric mucosa and serum in patients with gastritis and gastric ulcer: Does H. pylori. infection affect the mucosal levels. J Clin Gastroenterol 30: 381–385. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Nayan J, Janardhanan KK (2000): Antioxidant and antitumor activity of Pleurotus florida.. Curr Sci 79: 941–943. [CSA]
- Oh TY, Oh JS, Lee BO, Ahn H, Cho WB, Kim YJ, Surch SW, Hahm KB (2001): Oxidative damages are critical in pathogenesis of reflux esophagitis implication of antioxidants in its treatment. Free Radic Biol Med 30: 905–915. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Paya MB, Halliwell S, Hoult RS (1992): Interactions of a series of coumarins with reactive oxygen species. Biochem Pharmacol 44: 205–214. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Peterhans E (1997): Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation. J Nutr 127: 962–965. [CSA]
- Pluemaj T, Saifah E (1986): Constituents of Cissus quadrangularis. Linn. J Pharm Sci 11: 205–211. [CSA]
- Prashanth Kumar V, Shashidhara S, Kumar MM, Sridhara BY (2000): Effect of Luffa echnatia on lipid peroxidation and free radical scavenging activity. J Pharm Pharmacol 52: 891–894. [CSA]
- Raju K, Anbuganapathi G, Gokulakrishnan V, Rajkapoor B, Jayakar B, Manian S (2003): Effect of dried fruits of Solanum nigrum. Linn against CCl4-induced hepatic damage in rats. Biol Pharm Bull 26: 1618–1619. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ramanathan K, Shila S, Kumaran S, Pannerselvam C (2003): Ascorbic acid and α-tocopherol as potent modulators on arsenic induced toxicity in mitochondria. J Nutr Biochem 14: 416–420. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Rotruck JT, Pope AL, Ganther H, Awanson AB, Hafeman DG, Hoeckstra WG (1973): Selenium biochemical role as a component of glutathione peroxidase. Science 179: 588–590. [PUBMED], [INFOTRIEVE], [CSA]
- Sen SP (1964): Active constituents (oxo steroids) of C. quadrangularis.. Ind J Pharm 26: 247–248. [CSA]
- Shimada K, Fujikawa K, Yahara K, Nakamura T (1992): Antioxidative properties of xanthone on the auto oxidation of soybean in cylcodextrin emulsion. J Agric Food Chem 40: 945–948. [CSA], [CROSSREF]
- Shirwaikar A, Khan S, Malini S (2003): Antiosteoporotic effect of ethanolic extract of Cissus quadrangularis. L. on ovariectomized rat. J Ethnopharmacol 89: 245–250. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Singh ID (1980): In: Talwar GP, ed., Textbook of Biochemistry and Human Biology. New Delhi, Prentice Hall of India, pp. 152–153.
- Singh SP, Misra N, Dixit KS, Singh N, Kohli RP (1984): An experimental study of analgesic activity of Cissus quadrangularis.. Ind J Pharm 2: 162–163. [CSA]
- Singh B, Singh AK, Saxena BK, Chandan OP, Suri KA, Sathi NK (1998): Hepatoprotective activity of verbenalin on experimental liver damage in rodents. Fitoterapia 60: 135–138. [CSA]
- Sinha AK (1972): Colorimetric assay of catalase. Anal Biochem 47: 389–394. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sivarajan VV, Balachandran I (1994): Ayurvedic Drugs and Their Plant Sources. New Delhi, Oxford and India Book House Publishing Co., pp. 496–497.
- Sreeyan N, Rao M (1996): Free radical scavenging activity of curcuminoids. Drug Res 46: 169–171. [CSA]
- Sumathi R, Baskaran G, Varalakshmi P (1996): Effect of DL α-lipoic acid on tissue redox state in acute cadmium-challenged tissues. J Nutr Biochem 7: 85–92. [CSA], [CROSSREF]
- Tiangburanatham W (1996): Dictionary of Thai Medicinal Plants. Bangkok, Thailand, Prachumtong Printing, pp. 572–573.
- Udupa KN, Prasad G, Sen SP (1965): The effect of phytogenic anabolic steroid in the acceleration of fracture repair. Life Sci 4: 317–327. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Udupa KN, Chaturvedi GN, Tripathi SN (1970): Advances in Research in Indian Medicine, Vol. 12. Varnasi, Banaras Hindu University, pp. 165–196.
- Venukumar MR, Latha MS (2002): Antioxidant activity of curculigo orchoides in carbon tetrachloride-induced hepatopathy in rats. Ind J Clin Biochem 17: 80–87. [CSA]
- Wattarapenpaiboon N, Wahlqvist MW (2003): Phytonutrient deficiency: The place of palm fruit. Asia Pac J Clin Nutr 12: 363–368. [CSA]
