ABSTRACT
The ethanol root extract of Plumbago zeylanica. L. (PZE) was investigated for anti-inflammatory activity in adjuvant-induced arthritic (AIA) rats. The AIA rats were treated with PZE (20 mg/kg body weight) daily from day 0 to day 13. The PZE reduced the paw thickness and paw volume of AIA rats. The effect of PZE treatment was assessed in cutaneous delayed type hypersensitivity (DTH) response to Mycobacterium. tuberculin (MT) in development of AIA. The results demonstrated that PZE inhibited the development of cutaneous DTH response in AIA rats. The T-cell proliferation induced by concanavalin A (Con A), a mitogen, was depressed in three different strains of AIA rats (Wistar, Lewis, and Fischer) when compared with that of normal; however, the PZE treatment of AIA rats reversed the decrease of T-cell proliferation to the normal levels. The treatment of AIA rats with PZE on splenic T-cell proliferation was also compared with T-cell proliferation of rats that were treated with the known anti-inflammatory compounds such as cyclophosphamide and indomethacin.
Introduction
Plumbago zeylanica. L. (Plumbaginacae) is a perennial herb distributed throughout India, highly cultivated in gardens, and wild in the western peninsula and also in Bengal. A number of naphthoquinones, flavinoids, anthocyanins, and β-sitosterol have been reported previously from this plant source (Dinda et al., Citation1995). The chloroform extract exhibited antibacterial activity (Chakraborty, 1977). The impure extracts of Plumbago zeylanica. have been used in China and other Asian countries as folk medicine for the treatment of cancer, rheumatoid arthritis, and dysmenorrhea (Itoigawa et al., Citation1991). Plumbagin, a natural product isolated from this plant, was found to modulate the functions of peritoneal macrophages of BALB/c mice (Abdul & Ramchender, Citation1995). The immunomodulation by some other plant extracts have also been reported such as compounds isolated from Withania somnifera. (Srinivasulu Amara et al., Citation1999) and Achyranthus aspera. (Vasudeva Rao et al., Citation2002). The ethyl acetate extract and fraction, KSE-23, isolated chromatographically from Euphorbia prostrata. showed significant anti-inflammatory activity when topically applied in a murine model of carrageenan footpad edema, and it was found to be more potent than indomethacin given in the same manner (Singla & Pathak, Citation1990). The current study investigates the role of the alcohol extract of the roots of Plumbago zeylanica. (PZE) in using classical adjuvant-induced arthritic rat model (Trentham et al., Citation1977) of rheumatoid arthritis with reference to the induction of inflammation and T-cell proliferation.
Materials and Methods
Animals
Male Wistar and Fischer rats (6–8 weeks old) weighing around 180 g were procured from laboratory animal service center, National Institute of Nutrition (Hyderabad, India). Lewis rats weighing around 160 g were procured from the Experimental Animal Facility of National Institute of Immunology (New Delhi, India). The above-mentioned strains of rats were used for induction of adjuvant-induced arthritis and to study the effect of PZE in treatment of arthritis.
Chemicals and materials
Concanavalin A, complete Freund's adjuvant (CFA), incomplete Freund's adjuvant (IFA), trypan blue, RPMI-1640, Hank's balanced salt solution (HBSS), fetal calf serum (FCS), ammonium chloride, streptavidin, indomethacin, and cyclophosphamide were purchased from Sigma Chemical Company (St. Louis, MO, USA). The tritiated thymidine was procured from Baba Atomic Research Center (Mumbai, India) and the 96-well microtiter flat-bottom ELISA culture plates from Nunc (Denmark).
Preparation of crude extract
The plant, Plumbago zeylanica. (PZ), was identified and authenticated with the help of the taxonomist of the Department of Botany, Andhra University, and the roots were properly cleaned, and semi-dried in an air-circulating oven at temperature not exceeding 30°C. The dried roots were ground to prepare 15% aqueous extract. The extract was prepared by taking 15 g of material in 100 ml of double-distilled water then subjected to vigorous grinding to extract the contents of the material. The extract was lyophilized after vacuum filtration. The lyophilized material of Plumbago zeylanica. was weighed and stored at −20°C until its further use.
Purification of the extract
The lyophilized powder of Plumbago zeylanica. was separated into alcohol solubles (AS) and alcohol nonsolubles (ANS) depending on the polarity of the compounds. Briefly, the material was subjected to vigorous stirring with absolute alcohol until alcohol solubles were recovered. The alcohol solubles were separated by vacuum filtration using rotavapor. The left-over residue was allowed for hydration and dehydration by freeze drying to remove the traces of alcohol. Both the alcohol solubles and alcohol nonsolubles were tested for anti-inflammatory property. The ethyl alcohol soluble fraction of Plumbago zeylanica. was designated as PZE, which was found to be effective in inhibiting inflammatory response and modulating the T-cell response in AIA rats.
Induction of arthritis
Adjuvant arthritis was induced as described previously (Trentham et al., Citation1977). Briefly, rats of three different strains, namely, Wistar, Fischer, and Lewis (6–8 weeks old), were immunized with 0.1 ml of complete Freund's adjuvant (CFA) subcutaneously into the footpad of the right hind paw on day 0. The rats were found to develop adjuvant induced arthritis (AIA) by day 7. Control rats were injected similarly with 0.1 ml of incomplete Freund's adjuvant (IFA).
Treatment of PZE or other drugs
Two milligrams of PZE (dissolved in 0.5 ml of olive oil), cyclophosphamide, or indomethacin (suspended in 0.5 ml of carboxymethyl cellulose) were injected intraperitoneally into the adjuvant-induced arthritic rats or control rats from day 0 to day 13.
Assessment of adjuvant arthritis
The development of arthritis was studied on days 3, 7, 11, and 14 after CFA injection, and the following parameters are assessed. The daily administration of the PZE is continued until the 13th day as given below.
Paw thickness
Swelling of the hind paws of adjuvant arthritic rats and PZE-treated rats were quantitated by measuring the thickness of the ankle with a calipers.
Hind paw swelling by volume replacement
Plastic tubes were filled to the rim with water containing 1% detergent and weighed. Under light ether anesthesia, each hind paw was then immersed vertically into the plastic tubes up to the level of the line joining the medial and lateral malleolus of the ankle joint. The tubes were again weighed and the weight difference recorded as volume displaced. The changes in the hind paw volume are expressed as percentage of initial hind paw volume as it was on day 0 (Jones et al., Citation1982).
Clinical disease score
The incidence of arthritis was defined as the number of rats that had clinical evidence of arthritis within 14 days after the induction of disease. The severity of clinical disease was scored from 0 to 3 as follows: no swelling or limitation in movement = 0; distinct swelling of the distal hind limb joints but no limitation of movement = 1; moderate swelling of hind limb and fore limb joints and minor restriction of mobility = 2; marked swelling in all limbs and significant limitation in mobility = 3. The arthritis score for each rat was the sum of the score for each of the four paws. The maximum arthritis score was the highest score of an individual rat during the entire course of the disease.
Cutaneous delayed type hypersensitivity
Cutaneous delayed type hypersensitivity response (DTH) to Mycobacterium. tuberculin (MT) was assessed in adjuvant arthritic rats and PZE-treated rats. A small portion of the hair on the back of the animals was shaved and 50 µl of MT (0.05 mg) in oil or oil alone was injected intradermally 72 h prior to sacrifice. After the rats were sacrificed, on day 14, skin thickness was measured using calibrated skin fold calipers (Hallwell & Gutteridge Citation1981).
Assay of T-cell proliferation
For studying the effect of PZE on mitogen-induced T-cell proliferation, rats were injected subcutaneously with concanavalin A (Con A) on day 0, into the hind footpad with FCA, and booster dose was given on day 8 with IFA. On day 32, rats were sacrificed, and the spleens obtained from the rats that were treated with PBS were used as controls. Spleen cells that received PZE served as test groups.
The spleens from the control and treated rats were asceptically removed. The T cells were isolated according to the procedure of Francis et al. (1990). Briefly, the spleens were teased to get single cell suspension by incubating with 1 ml 0.9% NH4Cl for 1 min at 37°C, followed by adding RPMI twice the volume of the incubation mixture. The cells were washed thrice to remove the NH4Cl traces. Subsequently, the cells were suspended in RPMI and incubated in a Petri dish coated with goat anti-mouse immunoglobulins at 1:1000 dilutions in PBS to remove the B-cell population. The unbound cells were carefully separated and resuspended in fetal calf serum (FCS). The viability of cells was checked with trypan blue exclusion (Kaltenbach et al., 1990). These purified cells were employed in mitogen-induced T-cell proliferation assay.
T-cell proliferation and cell cultures were carried out in sterile microtiter plates (Nunc) with 96 flat-bottom wells. All the reagents used were filter sterilized using 0.22 µm Nunc millipore filters prior to adding into the wells. For each individual assay, the cultures were done in triplicate, with each well containing 2 × 105 cells in 250 µl RPMI medium. Cells were stimulated with mitogen (Con A) in absence or presence of different doses of extract. Cultures were incubated for 72 h at 37°C in a humidified 5% CO2 modulator chamber. After 18 h, the cultures were incubated with tritiated thymidine (1H3 0.5 µCi/well). After incubation period, cells were harvested on glass-fiber filters, using Nunc cell harvester, and the thymidine incorporation was determined by liquid scintillation counter (Wallac 1410, Pharmacia) by adding 5 ml of scintillation fluid [sulfur-free toulene containing 4.0 g of 2,5-diphenyloxazole (DPO) and 500 mg of 1,4-bis(5-phenyl-2-oxazole)-benzene; 2,2′-P-phenylene-bis(5-phenyl-oxazole) (POPOP)] per liter. The results were expressed as counts per minute (cpm) and plotted against the culture blanks, concentrations of the mitogen, and mitogen plus PZE.
Statistical analysis
Results were expressed as the average values with standard deviation (mean ± SD). Statistical significance was determined by two-way analysis of variance.
Results
Effect of PZE on inflammation in AIA rats
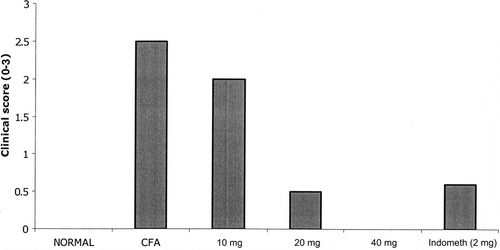
Adjuvant arthritis was induced using 6- to 8-week old rats of three different strains (Wistar, Fischer, and Lewis) by injecting 0.1 ml of CFA subcutaneously into the footpad of the right hind limb on day 0. The onset of arthritis was observed in rats (three strains) immunized with CFA on day 0 onwards. The earliest onset of disease was observed at day 9. Furthermore, 50% of rats had evidence of arthritis by day 11 and 100% by day 14 as evidenced by paw thickness measurement. As shown in , the treatment of AIA rats with PZE (20 mg/kg)significantly reduced the paw thickness measured on days 0, 3, 7, 11, and 14. Paw volume () and clinical score () measurements were taken on day 14 post administration of CFA.
Figure 1 Onset of arthritis was observed in three different strains of rats (Lewis, Wistar, and Fischer). Three strains of rats were injected with 0.1 ml of Freund's complete adjuvant into the footpad of right hind limb on day 0, and the development of arthritis was observed by measuring paw thickness on days 0, 3, 7, 11, and 14.
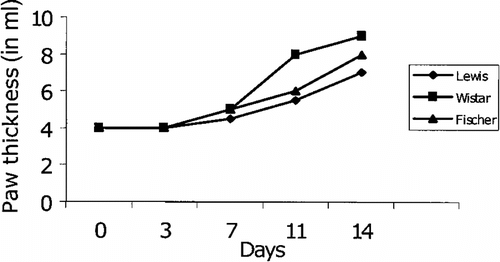
Figure 2 Effect of treatment with PZE at doses of 10, 20, and 40 mg/kg on paw volume on day 14 postadministration with adjuvant. Four groups of Lewis rats (5 rats per each group) were injected with 0.1 ml of Freund's complete adjuvant on day 0. Three groups of arthritic rats were treated (i.p.) with the above concentrations of PZE from day 0 to day 13. A fourth group (control) was treated with olive oil alone. Normal group did not receive any injection. Hind paw volume was measured on day 14.
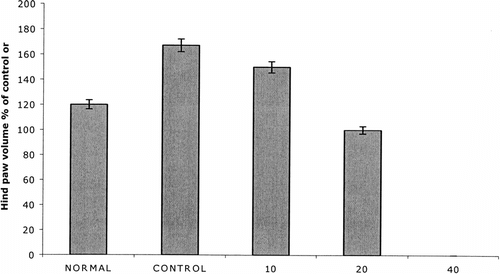
Figure 3 Five groups of Lewis rats were injected with 0.1 ml of Freund's complete adjuvant on day 0 to induce AIA. First group (control) was treated with olive oil alone. The four groups the rats were treated i.p. with PZE (10, 20, 40 mg) or indomethacin daily from day 0 to day 13. Normal group did not receive any treatment. Clinical score (0–3) was measured on day 14.
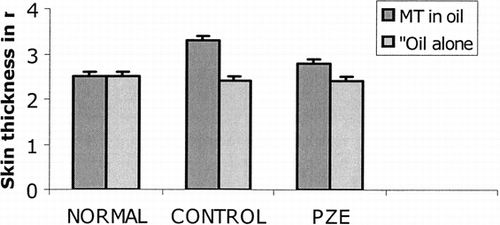
However, the results in demonstrate that PZE inhibited development of cutaneous DTH response in AIA rats. AIA rats developed increased skin thickness in response to challenge with MT in oil (3.3 ± 0.3 mm), which was significantly greater than the response to groups of rats that were given oil alone (2.4 ± 0.1 mm). The PZE has suppressed the cutaneous swelling in diseased rats to the same thickness to that of normal control rats (MT in oil 2.8 ± 0.6 mm, oil alone 2.4 ± 0.4 mm).
Figure 4 Effect of PZE (20 mg/kg) treatment on skin thickness after cutaneous challenge with mycobacterial antigens in mineral oil (CFA). Two groups of rats were injected with 0.1 ml of Freund's complete adjuvant (CFA) and another two groups of rats were injected with 0.1 ml of incomplete Freund's adjuvant (IFA). Two groups (one group from CFA injected + one group form IFA injected) were treated with PZE from day 0 to day 13. After the rats were sacrificed on day 14, skin thickness was measured using calibrated skin fold calipers. The group that received olive oil alone served as control. Normal group did not received any injection.
Comparision of the action of PZE, indomethacin, and cyclophosphamide on Con A–induced T-cell proliferation in AIA rats
Con A–induced T-cell proliferation was found to be decreased in three different strains (Wistar, Lewis, and Fischer) of arthritic rats tested. As shown in , results demonstrate that treatment of these groups with PZE enhanced the Con A–induced proliferation of T cells to the normal levels in adjuvant-induced arthritic rats. The effect of PZE treatment of AIA rats on splenic T-cell proliferation was compared with that of the known anti-inflammatory and immunosuppressive compounds such as indomethacin and cyclophosphamide. As seen in , the PZE restored mitogen-induced T-cell proliferation in AIA rats, whereas the indomethacin and cyclophosphamide could not bring the proliferative responses to the normal levels.
Figure 5 Splenic T lymphocytes were isolated from different strains (Lewis, Wistar, Fischer) of AIA rats, PZE-treated rats, or normal rats incubated with 10 µg of Con A (amitogen) and the conditions were maintained similar to the previous culture experiments. Cultures were pulsed with 0.5 µCi of radioactive thymidine per well, and the incorporation of radioactivity was measured in scintillation counter after 18 h of incubation.
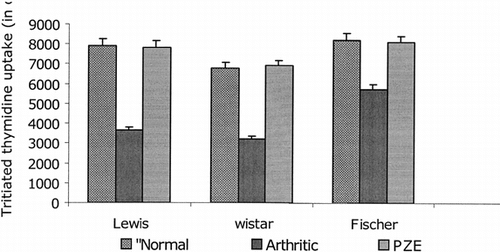
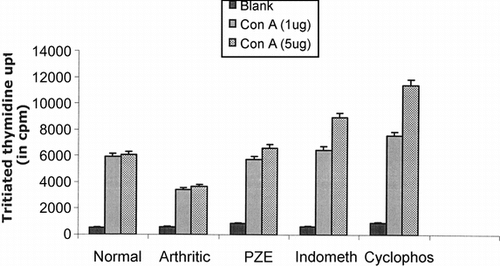
Figure 6 Five groups of Lewis rats were used. The first AIA group of rats was used as control without any treatment. The other three AIA groups of rats were treated with PZE, indomethacin (indometh) and cyclophosphamide (Cyclophos), respectively. Fifth group (control) received olive oil alone. Splenic T lymphocytes isolated from different strains of arthritic rats, PZE-treated rats, and normal rats incubated with 10 µg of Con A, and the conditions were maintained similar to the previous culture experiments. Cultures were pulsed with 0.5 µCi of radioactive thymidine per well, and the incorporation of radioactivity was measured in scintillation counter after 18 h of incubation.
Discussion
India with its wealth and variety of medicinal plants and the knowledge about their medicinal uses that has been accumulated over generations offers a great number of popular remedies for the treatment of rheumatoid arthritis (RA). Many of these are in common use even today. About 2000 plants of therapeutic value are mentioned in the Ayurvedic and Unani systems of medicine. Out of these, quite a large number of plants have been used in the alleviation of symptoms of arthritis. Some plants worth mentioning like Boerhaavia diffusa. (spreading hog weed), Commiphora mukul. (balsamodendron), Curcuma longa. (turmeric), and Glycirriza glabra. (liquorice), which have been scientifically screened, are still in common usage by Ayurvedic physicians. The modulation of immune response in the pathogenesis of RA by treatment with medicinal plants could provide an alternative to conventional chemotherapy. Resin extract of Boswellia serrata. has been used for the treatment of arthritis (Sander, Citation1998). Ginger is reported in the Ayurvedic and Tibbi systems of medicine to be useful in rheumatic disorders.
Our experimental data as shown in revealed that PZE suppressed the arthritis by decreasing inflammatory parameters such as footpad thickness, paw volume, clinical score, and delayed type hypersensitivity reaction (DTH). A significant suppression of arthritis by PZE was also observed in a dose-dependent manner. Inflammation is one of the characteristic features of RA. Oxygenation of arachidonic acid is increased in inflammed tissues. In this condition, products of two enzymatic pathways, the cyclooxygenase and 5-lipoxygenase producing respectively prostaglandins and leukotrienes, are elevated, which mediate inflammation in RA. Prolonged treatment with drugs like nonsteroidal anti-inflammatory drugs and corticosteroids causes adverse side effects (Srivastava & Mustafa, Citation1989). In order to minimize the side effects caused by the conventional therapy, the use of medicinal plants in the treatment of RA is practiced.
Furthermore, the alcohol soluble fraction of Plumbago zeylanica. (PZE) was tested for in vitro. mitogen-induced T-cell proliferation in AIA rats. As shown in , PZE restored mitogen-induced T-cell proliferation in AIA rats, whereas anti-inflammatory and immunosuppressive drugs such as indomethacin and cyclophosphamide could not bring it to the normal levels.
Conclusion
The above study thus establishes the anti-inflammatory and antiarthritic property of PZE. It may also have therapeutic applications in bronchial asthma, allergy, inflammation, arthritis, and various autoimmune diseases.
Acknowledgments
We thank Dr. Pramod Pal, research assosiate at AIIMS, New Delhi, for helping with the T-cell proliferation assay. We also thank UGC for their financial assistance.
References
- Abdul KM, Ramchender RP (1995): Modulation effect of plumbagin (5-hydroxy, 2-Methy 1,4-naphthoquinone) on macrophage functions in BALB/c mice. Potentiation of macrophage bactericidal activity. Immunopharmacol 30: 231–235. [CSA]
- Chakraborty RR, Patil AT (1997): Preliminary phytochemical and antimicrobial studies of Plumbago zeylanica.. Indian J Nat Prod 13: 3–7. [CSA]
- Chopra RN, Nayar SL, Chopra (1956): Glossary of Indian Medicinal Plants. New Delhi, India, Council of Scientific and Industrial Research.
- Dinda B, Das SK, Hajra AK (1995): Naphthoquinones from the roots of Plumbago rosea. Linn. Indian J Chem 34: 525–532. [CSA]
- Hallwell B, Gutteridge JM (1981): Formation of a thiobarbituric acid reactive substance from deoxyribose in the presence of iron salts. FEBS Lett 128: 347–354. [CSA], [CROSSREF]
- Itoigawa M, Takeya K, Furukawa H (1991): Cardiotonic action of plumbagin on guinea pig papillary muscle. Planta Med 57: 317–323. [PUBMED], [INFOTRIEVE], [CSA]
- Jones SA, Kennedy AJ, Roberts NA (1982): Assessment of drugs for activity in established type II collagen arthritis. Agents Actions 12: 5–12. [CSA], [CROSSREF]
- Kaltenbach JP, Kaltenbach MH, Lyons WB (1958): Nigrosin as a dye for differentiating live and dead ascites cells. Exp Cell Res 15: 112–116. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sander O, Herborn G, Rau R (1998): Is H15 (resin extract of Boswellia serrata., “incense”) a useful supplement to establish drug therapy of chronic poly arthritis? J Rheumatol 57: 11–16. [CSA], [CROSSREF]
- Saxena RS, Gupta B, Saxena KK, Singh RC, Prasad DN (1984): Study of anti-inflammatory activity in the leaves of Nyctanthes arboristris. linn. An Indian Medicinal Plant. J Ethnopharmacol 11: 319–323. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sethi PD, Thiagarajan AR, Subramanian SS (1970): Studies on the antiinflammatory and antiarthritic activity of withaferin A. Indian J Pharmacol 2: 165–170. [CSA]
- Singla AK, Pathak K (1990): Topical antiinflammatory effects of Euphorbia prostata. on carragenan induced footpad oedema in mice. J Ethnopharmacol 29: 291–303. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Srinivasulu Amara, Prasanna Kumar S, Rao R Athota (1999): Suppressive effect of withania somnifera. root extract on the induction of anti-OVA IgE antibody response in mice. Pharm Biol 37: 253–259. [CSA]
- Srivastava KC, Mustafa T (1989): Ginger (Zingiber officinals.) and rheumatic disorders. Med Hypothe Res 29: 25–29. [CSA], [CROSSREF]
- Trentham DE, Townes AS, Kang AH (1977): Autoimmunity to type II collagen: An experimental model of arthritis. J Exp Med 146: 857–861. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Vasudeva Rao Y, Govinda Rao D, Sunil Babu G, Rao R Athota (2000): Immunomodulatory properity of Achyranthus aspera. on elicitation of antigen specific murine humoral antibody response. Indian J Clin Biochem 42: 175. [CSA]
