ABSTRACT
Cadmium (Cd) is a ubiquitous metal and cumulative poison that may cause liver and kidney damage and the formation of neoplasia. The experiments were performed on white laboratory mice using intraperitoneal injections of 0.05 LD50 of cadmium chloride solution. Two groups of mice were injected with Echinacea purpurea. (L.) Moench liquid extract: one 0.05 LD50 and the other 0.1 LD50. Cd concentration in mouse blood, kidneys, liver, spleen, heart, and skeletal muscles was detected by atomic absorption spectroscopy. Estimated mitotic index of liver cells reflected the mitotic activity in the liver. Mitotic index in liver cells increases under the Cd intoxication, but it remains unchanged after simultaneous injection of Cd and Echinacea purpurea. liquid extract. In this study, the changes in organs and metabolism, the accumulation of various levels of intraperitoneal Cd in tissues, and the effects of Echinacea purpurea. liquid extract on Cd-induced changes in mice were investigated.
Introduction
Cadmium is a naturally occurring metal that is used in various chemical forms in metallurgy and for various industrial technologies, including the production of pigments. An environmental exposure can occur via diet and drinking water (ATSDR, 1989). Non-occupational exposure is mainly from cigarette smoke that contains relatively high concentrations of this element; for non-smokers who are not occupationally exposed, diet is the main route of exposure to cadmium (WHO, 1992).
Cadmium has a diversity of toxic effects including nephrotoxicity, carcinogenicity, teratogenicity, and endocrine and reproductive toxicity. Although Cd2+ is not essential for growth and development in mammals, it generally follows the metabolic pathways of the essential elements zinc and copper (Anon., 1995). One reason for this is that cadmium can act as an antagonist to zinc in zinc-requiring metalloenzymes, such as carbonic anhydrase (Lindskog & Malmstram, 1962; Lewis et al., Citation1972), alkaline phosphates (Plocke et al., Citation1962; Lewis et al., Citation1972), and others (Vallee, Citation1959; Lewis et al., Citation1972). Cadmium is a potent animal carcinogen (Sunderman, Citation1978) and has recently been upgraded to a human carcinogen by the International Agency for Research on Cancer (Anon., 1993).
Metabolic transformations of Cd2+ are limited to its binding to the protein and nonprotein sulfhydryl groups, and various macromolecules, such as metallothionein, which is especially important in the kidneys and liver (ATSDR, 1989). Cd2+ is excreted primarily in the urine. Cd2+ is detoxified by long-term tissue storage. The biological half-life of Cd2+ in humans is 10–30 years. Longer term exposure to Cd2+ primarily affects the kidneys, resulting in tubular proteinosis, although other conditions such as “itai-itai” disease may involve the skeletal system. (Goyer, Citation1991). Echinacea purpurea. (L.) Moench (EP) has many beneficial features, especially strengthening the immune system (Grimm & Muller, Citation1999; Abdullah, Citation2000). Many chemical compounds with pharmacological activity have been identified in Echinacea. species; however, attempts to isolate a single compound responsible for the therapeutic effects of the herb have not been successful (Foster, Citation1996). The broad chemical composition suggests a synergistic effect among the constituents. Three classes of phytochemicals found in Echinacea purpurea. that are thought to stimulate the immune system include polysaccharides (arabinogalactan), alkylamides (isobutylamides), and phenolics (which includes caffeic acid derivatives such as cichoric acid) (Bone, Citation1997). Overall, researchers have concluded that the total immunostimulatory activity of alcoholic and aqueous Echinacea. preparations depends on the combined action of several constituents (Snow, Citation1997). This study investigated the changes in organs and metabolism, the accumulation of various levels of intraperitonial Cd2+ in tissues, and the effects of Echinacea purpurea. liquid extract on Cd2+-induced changes in mice.
Materials and Methods
Experiments were carried out on the white laboratory mice whose weight was 20–25 g. Experimental groups were used as follows:
The Cd group. It was composed of mice intoxicated by Cd2+ (n = 10). CdCl2 solution (0.05 LD50) in twice-distilled water (0.16 mg Cd2+ per 1 kg of body mass) was used for this purpose. The solution was periodically injected into the peritoneum of the mice for 6 weeks 3 times per week.
EP group. The mice were injected by EP liquid extract only. Injections were made according to the same scheme. For this purpose, two concentrations of the EP liquid were used: 0.05 LD50 (0.125 g Echinacea. per 1 kg of body mass) (n = 9), and 0.1 LD50 (0.25 g Echinacea. per 1 kg of body mass) (n = 9).
Cd + EP group. The group intoxicated by Cd and injected by EP liquid. Injections were made according to the same scheme. In addition to the CdCl2 solution (0.05 LD50), liquid extract of EP 0.05 LD50 (n = 13) and EP 0.01 LD50 (n = 10) was injected.
Control mice (n = 9) were injected by the same volume of 0.9% saline.
Mice were anesthetized and sacrificed 6 weeks after Cd intoxication according to the rules defined by the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (license no. 0028). We measured Cd concentration in EP liquid extract. The concentration of Cd in blood and tissue specimens taken from liver, kidneys, heart, spleen, skeletal muscle, and EP liquid extract was determined using an electrothermal graphite furnace atomic absorption spectrophotometer (Perkin-Elmer/Zeeman 3030). The venous blood was obtained by single-use syringes using anticoagulant. Tissue specimens were resolved with 0.125 M NaOH upon 90°C, and the digests were diluted to the appropriate volume with twice-distilled water. The modified analysis method (Schlemmer, Citation1989) for heavy metals concentration detection in biological samples was used.
The preparation of extract from Echinacea purpurea.
The raw material of Echinacea purpurea. (Pharmeuropa, 2002) was imported from Poland. Maceration and percolation was used to prepare an extract from dried E. purpurea..
One kilogram of the dried herb of Echinacea purpurea. was ground into particles sized 1 mm. The herb was evenly moistened with 1000 ml of 40% aqueous ethanol (menstruum) and then placed in a closed vessel for 24 h. The moist herb was then introduced into the percolator. The percolator was placed in position, and a soak of 40% aqueous ethanol (1 l) was poured on (to make a 1:2 extract). Percolation continued for 24 h at the flow speed of extraction being 0.2 ml/min. When the percolation was finished, the marc (the inert fibrous material) of Echinacea purpurea. was removed from the percolator and pressed, and the expressed liquid was then mixed with the percolate. The 1:1 liquid extract was made using a concentration step. The resulting percolate was then filtered (Anon., 1996). One milliliter of extract contained 0.089 g of the dried herb of Echinacea purpurea..
Histology
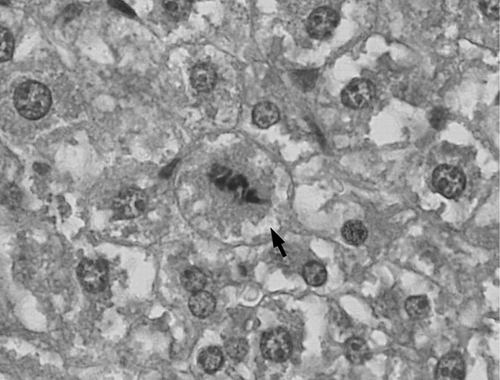
Samples from liver tissue were fixed in 10% neutral buffered formalin for 48 h and then processed for routine paraffin embedding. Five-micrometer-thick sections were routinely stained with hematoxylin and eosin. Histological slides were examined by light microscopy (objective × 40), (). For each specimen, the number of mitotic cells was counted in 10 randomly selected reference areas (0.04 mm2). Their histological images were taken using Olympus Digital Camera DP-11.
Statistical analysis
Nonparametric Kruskal-Wallis and Mann-Whitney tests were applied for evaluation of difference among mitotic cells counts in different groups. The Student's t.-test with Bonferroni correction was applied for comparison of geometric means of cadmium concentration. Statistical significance was set at p = 0.05.
Results
Cd2+ concentration in all organs of the mice in the control group was from 0.0008 µg/g in the skeletal muscles to 0.2063 µg/g in the kidneys. Cd2+ concentration in the blood was 0.0034 µg/dl. The range of Cd2+ concentration in all organs of mice injected only with EP 0.05 LD50 was from 0.0016 µg/g in the skeletal muscles to 0.078 µg/g in the kidneys, and that in mice injected with EP 0.1 LD50 was 0.0016 to 0.0658 µg/g. Cd2+ concentration in blood was 0.0286 µg/dl and 0.0133 µg/dl, respectively () There was no significant difference in Cd2+ concentration in the organs between the control and the EP groups.
Table 1. Geometric mean and 25th–75th percentiles of Cd concentration in various organs and blood of mice from the control group and those injected by Echinacea purpurea. (L.) Moench liquid extract 0.05 LD50 (EP 0.05) and 0.1 LD50 (EP 0.1).
Cd2+ concentration was also measured in EP extract liquid. There was 0.00362 µg Cd in 1 ml of the extract.
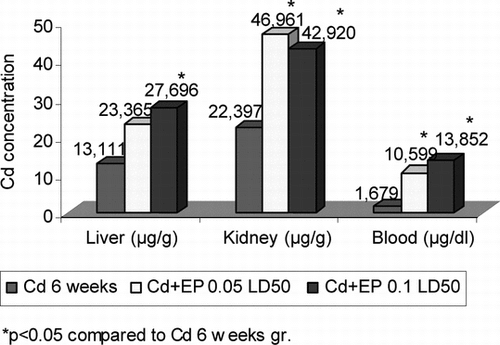
Periodical injection of CdCl2 for 6 weeks led to a significant increase in Cd2+ concentration in all organs and blood of the mice. This concentration ranged from 0.245 µg/g in the skeletal muscles to 22.397 µg/g in the kidneys ( and ). It is interesting to note that the injection of CdCl2 together with EP caused a significant increase in Cd2+ concentration in the liver, kidneys, spleen, and blood compared with the Cd group, while such tendency was detected only in the heart and skeletal muscles. Cd2+ concentration in the studied organs of Cd + EP group did not depend on the amount of EP. Spleen of mice injected with EP 0.05 LD50 contained 1.88-fold and injected with EP 0.1 LD50 contained 1.86-fold higher Cd2+ concentration, liver 1.78-fold and 2.11-fold, kidneys 2.09-fold and 1.92-fold, and blood 6.31-fold and 8.25-fold higher Cd2+ concentration.
Figure 2 Cd2+ concentration in heart, spleen, and muscle of mice after CdCl2 (0.16 mg Cd/1 kg body mass) and EP (0.125 EP extract/1 kg body mass and 0.25 EP extract/1 kg body mass) injection.
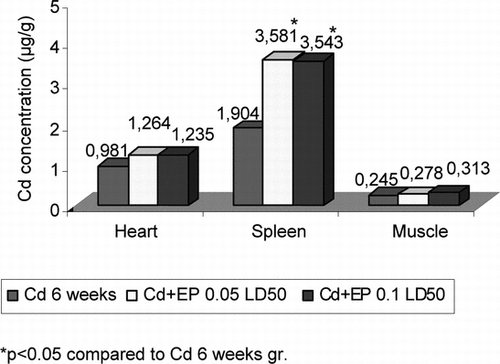
Figure 3 Cd2+ concentration in blood, liver, and kidneys of mice after CdCl2 (0.16 mg Cd/1 kg body mass) and EP (0.125 EP extract/1 kg body mass and 0.25 EP extract/1 kg body mass) injection.
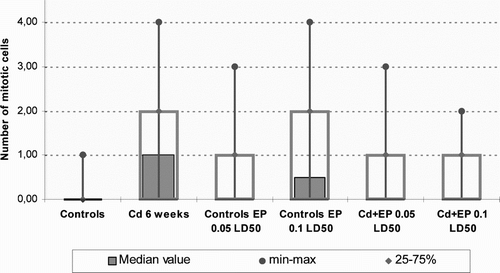
Cd2+ concentration in the studied organs of Cd + EP group did not depend on the amount of EP. Mitotic activity of liver cells () was evaluated by calculating the mitotic index (MI). As compared with the control group, it was statistically significantly higher after Cd2+ injection. The increase in MI after EP 0.1 LD50 injection was not significant. In Cd + EP groups, MI remained the same as in the control group.
Discussion
The results of this study showed that 6 weeks of periodical parenteral administration of CdCl2 for the mice led to the increase in Cd2+ concentration in liver, kidneys, spleen, and blood, as compared with the controls. Once absorbed, the clearance of cadmium from the circulation and deposition into the tissue is rapid. More than 50% of the body burden of cadmium is localized in liver and kidneys (Bernard & Lauwerys, Citation1986). Nordberg et al. (Citation1985) indicated that soon after Cd2+ parenteral injection, 95% of Cd2+ was found in the plasma of experimental animals, in the structure of a-globulin, albumin, and other proteins of 48–77 kDa molecular weight. Within a short time, blood plasma lost a large amount of Cd2+. Cd2+connected to metallothioneins remained in the plasma. The binding of cadmium to metallothioneins prevents the free cadmium ions from exerting their toxic effects. Metallothioneins will bind cadmium with high affinity, and the intracellular cadmium-metallothionein (Cd-MT) complex is relatively biologically inert.
Our results indicate that long-term administration of CdCl2 leads to the highest hepatic and renal concentration of Cd2+. The remarkable capacity of the liver and kidneys to synthesize metallothioneins is responsible for the high accumulation of cadmium in these organs. In acute exposure, most of the cadmium is distributed to the liver, but redistribution to the kidneys occurs after hepatic production of metallothionein (Nordberg, Citation2000). The induction of the synthesis of metallothioneins prior to the exposure to toxic or lethal doses of cadmium will induce tolerance to these effects (Goering & Klaassen, Citation1983). Cadmium is detoxified to a degree by long-term tissue storage. The biological half-life of cadmium in humans is 10–30 years (Goyer, Citation1991). Consequently, with continuous environmental exposure, the concentrations of cadmium in tissues will increase slowly throughout life. Cadmium is a toxic divalent cation that can initiate either mitogenic signals or apoptosis, possibly as a consequence of inducing different patterns of oncogene expression in different cells (Wang & Templeton, Citation1998). In the current study we also noticed a positive shift in the mitotic index in the mice intraperitoneally injected with CdCl2. That may be linked to the replenishment of liver cells, arising out of the toxic CdCl2 effect to chromosome components primarily made up of DNA and protein. The repair mechanisms of chromosomes, therefore, also involved inherent mechanisms for repair of protein damage, a process that is also under a precise genetic control (Cooper, Citation1997; Lewin, Citation1997; Gardner & Snaustad, Citation1984). MI remains unchanged when mice are injected with different concentration of EP extract during Cd intoxication.
Our study for the first time revealed that the administration of herbal extract of Echinacea purpurea. together with CdCl2 increased cadmium concentration in the blood and organs. Most of the studies carried out with Echinacea purpurea. are oriented around the immune system. Researchers have concluded that the total immunostimulatory activity of alcoholic Echinacea. preparations depends on the combined action of several constituents (Snow, Citation1997). Among the numerous compounds that have been identified, the alkylamides, cichoric acid derivatives, polysaccharides (arabinogalactan), and possibly glycoproteins are the most promising. Other major groups of compounds include flavonoids, monoterpenes, hydrocarbons such as n.-alkanes, polyacetylenes, and traces of pyrrolizidine alkaloids (Parnham, Citation1996; Schulz et al., Citation1998).
It was also revealed that Echinacea purpurea. has many metal-binding substances. Among them are cichoric acid derivatives, polysaccharides (arabinogalactan), glycoproteins, flavonoids, and others. These compounds that exhibit high chelating properties may also constitute an antidote to heavy metals in vivo. (Clifford, Citation1999; Kroon & Williamson 1999; Soczynska-Kordala et al., 2001). Echinacea. has been shown to produce a profound effect on the immune system as an immunostimulant. One of the effects of EP is the activation of macrophages to produce IL-1, TNF-µ, and IL-6 (Luettig et al., Citation1989; Melchart et al., Citation1994). It is known that metallothionein is directly induced by cytokines, and it accumulates in the liver, kidneys, spleen, and—to a lesser extent—in other organs (Sato et al., Citation1995). It can be speculated that the administration of EP leads to the increased amount of metallothionein and chelators in the organs. Thus it leads to the higher accumulation of cadmium in these organs during chronic intoxication.
We are not currently able to describe the precise mechanism of the presented phenomenon, that is, that the administration of Cd together with EP leads to higher concentration of Cd in the internal organs and blood as compared with the intoxication by Cd alone. The explanation of this phenomenon requires more detailed investigation in order to reveal which mechanisms are responsible.
References
- Abdullah T (2000): A strategic call to utilize Echinacea-garlic in flu-cold seasons. J Natl Med Assoc 92: 65. [CSA]
- Anonymous (1993): Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. In: International Agency for Research on Cancer Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, IARC Scientific Publications, pp. 119–237.
- Anonymous (1995): Toxicology of metals. In: Biochemical Aspects. Berlin, Heidelberg, Springer, p. 189.
- Anonymous (1996): British Herbal Pharmacopoeia. Bournemouth, UK, British Herbal Medicine Association's Scientific Committee.
- ATSDR (Agency for Toxic Substances and Disease Registry) (1989): Toxicological profile for cadmium. U.S. Public Health Service.
- Bernard A, Lauwerys R (1986): Effects of cadmium exposure in man. Cadmium Toxicology. Berlin, Heidelberg, New York, Springer, pp. 135–177.
- Bone K (1997): Echinacea: what makes it work? Alt Med Rev 2: 87–93. [CSA]
- Clifford MN (1999): Chlorogenic acids and other cinnamates—nature, occurrence, dietary burden, absorption and metabolism. Guildford, Surrey, School of Biological Sciences, University of Surrey.
- Cooper GM (1997): The Cell—A Molecular Approach. Washington, DC, ASM Press.
- Foster S (1996): Echinacea: The Purple Coneflowers. Botanical Series No. 301. Austin, American Botanical Council.
- Gardner EJ, Snaustad DP (1984): Principles of Genetics. New York, John Wiley & Sons.
- Goering PL, Klaassen CD (1983): Altered subcellular distribution of cadmium following cadmium pretreatment: Possible mechanism of tolerance to cadmium-induced lethality. Toxicol Appl Pharmacol 70: 195–203. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Goyer R (1991): Toxic effects of metals. In: Casarett and Doull's Toxicology. New York, Pergamon Press, pp. 623–680.
- Grimm W, Muller HH (1999): A randomised controlled tial of then effect of fluid extract of Echinacea purpurea. on the influence and severity of colds and respiratory infections. An J Med 106: 138–143. [CSA], [CROSSREF]
- Kroon PA, Williamson G (1999): Hydroxycinnamates in plants and food: Current and future perspectives. JSFA 79: 355–361. [CSA], [CROSSREF]
- Lewin B (1997): Genes VI. New York, Oxford University Press.
- Lewis GP, Coughlin LL, Jusko JW, Hartz S (1972): Contribution of cigarette smoking to cadmium accumulation in man. Lancet 1: 291. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lindskog S, Malmstrom BG (1962): Metal binding and catalytic activity in bovine carbonic anhydrase. J Biol Chem 237: 1129. [PUBMED], [INFOTRIEVE], [CSA]
- Luettig Bet al. (1989): Macrophage activation by polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea.. JNCI 81: 669–675. [PUBMED], [INFOTRIEVE], [CSA]
- Melchart D , et al. (1994): Immunomodulation with Echinacea—a systematic review of controlled clinical trials. Phytomedicine 1: 245–254. [CSA]
- Nordberg GF, Kjellstrom T, Nordberg M (1985): Kinetics and Metabolism. A Toxicological and Epidemiological Appraisal. Boca Raton, CRC Press, pp. 103–178.
- Nordberg M (2000): Trace elements in human health and disease: An Update. J Trace Elements Exp Med 13: 97–04. [CSA], [CROSSREF]
- Parnham MJ (1996): Benefit-risk assessment of the squeezed sap of the purple coneflower (Echinacea purpurea.) for long-term oral immunostimulation. Phytomedicine 3: 95–102. [CSA]
- Pharmeuropa (2002): 14: 138–140. [CSA]
- Plocke DJ, Levinthal C, Vallee BL (1962): Alkaline phosphatase of Escherichia coli: A zinc metalloenzyme. Biochemistry 1: 373. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sato M, Sasaki M, Hojo H (1995): Antioxidative roles of metallothionein and manganese superoxide dismutase induced by tumor necrosis factor-alpha and interleukin-6. Arch Biochem Biophys 316: 738–744. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Schlemmer G (1989): Analyse von biologishem Material mit der Graphitrohrofen – AAS. Instrumentalized Analytical Chemistry and Computer Technology. Darmstadt, Git Verlag, pp. 561–568.
- Schulz V, Hansel R, Tyler VE (1998): Rational Phytotherapy: A Physician's Guide to Herbal Medicine. New York, Springer, pp. 273–278.
- Snow JM (1997): Echinacea (Moench) Spp. Asteraceae. Protocol J Botan Med 2: 18–24. [CSA]
- Soczynska-Kordala M, Bakowska A, Oszmianski J, Gabrielska J (2001): Metalion-flavonoid associations in bilayer phospholipid membranes. Cell Mol Biol Lett 6(2 A): 277–281. [PUBMED], [INFOTRIEVE], [CSA]
- Sunderman FW (1978): Carcinogenic effects of metals. Fed Proc 37: 40–46. [PUBMED], [INFOTRIEVE], [CSA]
- Vallee BL (1959): Biochemistry, physiology and pathology of zinc. Physiol Rev 39: 443. [PUBMED], [INFOTRIEVE], [CSA]
- Wang Z, Templeton DM (1998): Induction of c-fos. proto-oncogene in mesangial cells by cadmium. J Biol Chem 273: 73–79. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- WHO (1992): Cadmium. Environmental Health Criteria 134. Geneva, World Health Organization.