Abstract
The methanol extract of Randia hebecarpa. Benth. (Rubiaceae) leaves was evaluated for anti-inflammatory activity using carrageenan- and dextran-induced rat paw edema models. Antioxidant activities of the methanol extract and of the fractions resulting from its partition were also measured using the 1,1-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay and the linoleic acid peroxidation method. The methanol extract, ethyl acetate fraction, and hydromethanol fraction exhibited percent inhibition of lipid peroxidation comparable to that of commercial antioxidant butylated hydroxytoluene (BHT). Fractionation of the ethyl acetate and hydromethanol fractions through chromatographic methods yielded kaempferol-3, 7-O.-α.-L-dirhamnoside, kaempferol-3-O.-β.-D-galactoside, quercetin-3-O.-β.-D-galactoside, myricetin-3-O.-α.-L-rhamnoside, kaempferol-3-O.-α.-L-rhamnosyl-(1 → 6)-β.-D-galactosyl-7-O.-α.-L-rhamnoside, quinovic acid-3-O.-β.-D-quinovopyranosil-28-O.-β.-D-glucopyranoside, cincholic acid 3-O.-β.-D-quinovopyranosil-28-O.-β.-D-glucopyranoside, and the sugar D-mannitol.
Introduction
Randia hebecarpa. Benth. (Rubiaceae) is a small shrub that grows in South America. In Brazil, this species is popularly known as “limãozinho.” or “quina-branca.” and it is used as a medicinal plant (Tanaka, Citation2001). However, no report on its biological or chemical investigation has been found in the literature. Species of the genus are known to contain iridoids (Useato et al., Citation1982; Hamerski et al., Citation2003), and some of them display anti-inflammatory activity (Recio et al., Citation1994) and biologically active saponins (Sati et al., Citation1989; Dubois et al., Citation1990; Jansakul et al., Citation1999; Sahpaz et al., Citation2000).
In the current study, we have evaluated the anti-inflammatory, free radical scavenging, and lipid peroxidation inhibition activities of the methanol extract of Randia hebecarpa. (leaves). Antioxidant assays of the hexane, ethyl acetate, and hydromethanol fractions resulting from the partition of the crude extract were also performed. The major chemical constituents of the ethyl acetate and hydromethanol fractions were isolated through chromatographic fractionation and characterized by NMR spectral data.
Materials and Methods
Plant material
Randia hebecarpa. leaves were collected in Porto Rico-Pr, Brazil, in December 2001. A voucher specimen (HNUP 2124) is deposited at the herbarium of Universidade Estadual de Maringá.
Extraction and isolation
Air-dried leaves of R.. hebecarpa. (300 g) were exhaustively extracted by maceration with 95% methanol. The methanol extract (22 g) was dissolved in MeOH–H2O 1:1 and partitioned with n.-hexane and ethyl acetate. The ethyl acetate fraction (2.04 g) was subjected to chromatographic column on silica gel (30 g; 70–230 mesh) eluted with CHCl3,CHCl3–MeOH (9:1, 8:2, 6:4, 1:1), and MeOH to give 61 fractions. The grouped fractions (15–21) and (29–34) were purified on Sephadex LH-20 by using H2O, H2O–MeOH (1:1) and MeOH. Recrystallization of the subfractions obtained afforded kaempferol-3, 7-O.-α.-L-dirhamnoside (11.7 mg), a mixture of kaempferol-3-O.-β.-D-galactoside and quercetin-3-O.-β.-D-galactoside (6.1 mg), myricetin-3-O.-α.-L-rhamnoside (4.2 mg), quinovic acid-3-O.-β.-D-quinovopyranosil-28-O.-β.-glucopyranoside (5.2 mg), and cincholic acid-3-O.-β.-D-quinovopyranosil-28-O.-β.-D-glucopyranoside (6.4 mg). The hydromethanol fraction resulting from the partition of the methanol extract afforded a brown residue after concentration. Addition of methanol to this residue resulted in two fractions: a MeOH-soluble fraction and a MeOH-insoluble fraction. Purification of the MeOH-soluble fraction on Sephadex LH-20 afforded kaempferol-3-O.-rhamnosyl-(1 → 6)-O.-β.-D-galactosyl-7-O.-β.-D-rhamnoside (10.6 mg). Crystallization of the MeOH-insoluble part (chloroform-MeOH) yielded D-mannitol (23.8 mg).
NMR spectra were recorded on a Varian Mercury Plus BB spectrometer (Palo Alto, CA, USA) by using CD3OD as a solvent and TMS as an internal standard.
Anti-inflammatory activity assay
Carrageenan- and dextran-induced rat paw edema
The methanol extract of R. hebecarpa. (600 and 1000 mg kg−1), the vehicle (DMSO solution 15%), and a saline solution (0.9% w/v NaCl) were administered by oral route 30 min before injection of 0.1 ml of either carrageenan (2.0 mg/ml) or dextran (1.0 mg/ml) to the subplantar area of the right hind paw. The volume (ml) of the paw was determined with a plethysmometer as described by Winter et al. (Citation1962). The volume was measured before injection of carrageenan and dextran and after 1, 2, and 4 h. The edema was reported as the difference between the final and initial paw volume. The anti-inflammatory effect was expressed as the percentage inhibition caused by the extract in comparison with vehicle-treated animal. Edema values are expressed as mean ± SEM. The statistical significance of the changes was analyzed by Student's t.-test. p < 0.05 was considered significant.
Determination of antioxidant activity
Linoleic acid peroxidation method
The inhibition of linoleic acid peroxidation was determined by using the ferric thiocyanate method (Osawa & Namiki, Citation1981). The samples (2.5 mg) were mixed with 2.51% linoleic acid in 99.5% ethanol (5 ml) and 0.2 M phosphate buffer (pH 7.0, 5 ml). The solutions were incubated at 37°C in a screw-cap glass vial in darkness. During incubation, an aliquot of 0.1 ml of each solution was diluted with 10 ml of 75% ethanol, and 0.1 ml of 30% ammonium thiocyanate and 0.2 ml of ferrous sulfate solution (20 mM in 3.5% HCl) were added. After stirring for 3 min, absorbance was measured at 500 nm. The solutions without added extracts were used as blanks. Butylated hydroxytoluene (BHT) was used as a positive control. All data are the averages of triplicate analyses.
DPPH free radical scavenging
The free radical scavenging activity was measured using the 1,1-diphenyl-1-picrylhydrazyl (DPPH) assay (Cho et al., Citation2003). Various sample concentrations were added to 3 ml of daily-prepared methanol DPPH solution (0.1 mM). The mixture was shaken and left to stand at room temperature in the dark. After 30 min, absorbance was measured at 517 nm. Assays were carried out in triplicate.
Results and Discussion
The methanol extract of Randia hebecarpa. (leaves) did not show significant reduction of inflammation provoked by injection of carrageenan or dextran to the hind paw of rats. Assays using isolated compounds revealed that flavonoids exhibit different anti-inflammatory action in different inflammation models (Rotelli et al., Citation2003). Therefore, our results suggest that the anti-inflammatory activity of the extract can be directly related to the experimental models used.
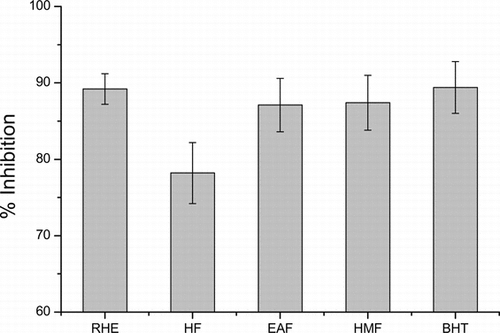
The effects of the methanol extract and its fractions on the linoleic acid peroxidation are shown in . The percent inhibition of linoleic acid peroxidation was calculated using the following equation: % Inhibition = (A0 − A1)/A0 × 100), where A0 is the absorbance of the control and A1is the absorbance in the presence of test samples. The results show that methanol extract (89.2% of inhibition), ethyl acetate fraction (87.1%), and hydromethanol fraction (87.4%) presented antioxidant activity comparable to that of BHT (89.4%).
Figure 1 Inhibition (%) of lipid peroxidation for the methanol extract (RHE), hexane (HF), ethyl acetate (EAF), and hydromethanol (HMF) fractions measured by a ferric thiocyanate (FTC) test (mean ± SD; n = 3) compared with butylated hydroxy toluene (BHT).
The IC50 values (µg/ml) for 50% of DPPH scavenging radicals are shown in . The data showed that the ethyl acetate fraction (IC50 = 60.8 µg/ml) exhibited the highest free radical scavenging activity when compared with methanol extract, and with hexane and hydromethanol fractions.
Table 1 DPPH free radical scavenging (IC50) of RHE extract and HF, EAF, and HMF fractions of Randia hebecarpa..
The most active ethyl acetate and hydromethanol fractions were subjected to fractionation through chromatographic techniques to give five flavonoids, two saponins, and one sugar. The structures of isolated compounds were identified by analysis of their NMR spectra and comparison with literature data (Agrawal et al., Citation1989; Arriaga et al., Citation1990; Yahara et al., Citation2000). The nature of the sugar moieties, as well as the position of their linkage to the aglycone, was determined by a combination of 2D NMR experiments (1H–1H COSY, NOESY, and HMBC). The coupling constant values for the anomeric hydrogens were used to establish the β-configuration for D-glucopyranose and D-galactopyranose and the α-configuration for L-rhamnopyranose.
These findings demonstrate that the antioxidant activity of the plant can be attributed to the presence of flavonoids. The DPPH free radical scavenging activity of quercetin-3-O.-β-D-galactoside (Cho et al., Citation2003) and lipid peroxidation inhibition of myricetin-3-O.-α-L-rhamnoside (Hopia & Heinone, Citation1999), both compounds isolated in the current work, have been described in literature.
Acknowledgments
We are grateful to CAPES and CNPq for fellowships (A.S.N. and S.A.D.) and to Fundação Araucária (Paraná State, Brazil) for financial support.
References
- Arriaga FJ, Rumbero A, Vasquez P (1990): Two triterpene glycosides from Isertia haenkeana.. Phytochemistry 29: 209–213, [CSA]
- Agrawal PK, Bansal MC (1989): Flavonoid glycosides. In: Agrawal PK, ed., Carbon-13 NMR of Flavonoids. New York, Elsevier, pp. 334–337.
- Cho EJ, Yokozawa T, Rhyu DY, Kim SC, Shibahara N, Park JC (2003): Study on the inhibitory effects of Korean medicinal plants and their main compounds on the 1,1-diphenyl-2-picrylhydrazyl radical. Phytomedicine 10: 544–551, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Dubois MA, Benze S, Wagner H (1990): New biologically active triterpene saponins from Randia dumetorum.. Planta Med 56: 451–455, [PUBMED], [INFOTRIEVE], [CSA]
- Hamerski L, Furlan M, Silva DHS, Cavalheiro AJ, Eberlin MN, Tómasela DM, Bolzani VS (2003): Iridoid glucosides from Randia spinosa. (Rubiaceae). Phytochemistry 63: 397–400, [PUBMED], [INFOTRIEVE], [CSA]
- Hopia A, Heinonen M (1999): Antioxidant activity of flavonol aglycones and their glycosides in methyl linoleate. J Am Oil Chem Soc 76: 139–144, [CSA]
- Jansakul C, Intarit K, Itharat A, Phadungcharoen T, Ruangrungsi, N, Merica A, Lange GL (1999): Biological activity of crude extract and saponin pseudoginseniside-RT1 derived from the fruit of Randia siamensis.. Pharm Biol 37: 42–45, [CSA]
- Osawa T, Namiki K (1981): A novel type of antioxidant isolated from leaf wax of Eucalyptus leaves. Agr Biol Chem 45: 735–739, [CSA]
- Recio MC, Giner RM, Mnez S, Rios JL (1994): Structural considerations on the iridoids as ani-inflammatory agents. Planta Med 60: 232–234, [PUBMED], [INFOTRIEVE], [CSA]
- Rotelli AE, Guardia T, Juarez AO, Rocha NE, Pelzer LE (2003): Comparative study of flavonoids in experimental models of inflammation. Pharmacol Res 48: 601–606, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Sahpaz S, Gupta MP, Hostettman K (2000): Triterpene saponins from Randia formosa.. Phytochemistry 54: 77–84, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Sati OP, Bahuguna S, Uniyal S, Bhakuni DS (1989): Triterpenoid saponins from Randia uliginosa.. Phytochemistry 28: 575–577, [CROSSREF], [CSA]
- Tanaka I (2001): Plantas medicinais de uso popular na planície de inundação do alto rio Paraná, região de Porto Rico (Paraná e Mato Grosso do Sul). MSc thesis, Postgraduate Programme in Ecologia de Ambientes Aquáticos Continentais, Universidade Estadual de Maringá, Paraná, Brazil.
- Useato S, Ali E, Nishimura H, Kawamura I, Inouye H (1982): Studis on monoterpene glucosides and related natural products. 4 Iridoids from Randia canrhioides.. Phytochemistry 21: 353–357, [CROSSREF], [CSA]
- Winter C, Risley E, Nuss G (1962): Carrageenan-induced edema in hind paw of rats as an assay for anti-inflammatory drug. Proc Soc Exp Biol Med 111: 207–210, [CSA]
- Yahara S, Kohjyouma M, Kohoda H (2000): Flavonoid glycosides and saponins from Astragalus shikokianus.. Phytochemistry 53: 469–471, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
