ABSTRACT
The antimicrobial activity of different extracts of Tagetes lucida. Cav. (Asteraceae) against 11 bacterial strains and one yeast strain (Candida albicans.) was evaluated. The ethyl acetate extract showed antibacterial activity against Shigella boydii., Staphylococcus aureus., Staphylococcus epidermidis., Pseudomonas aeruginosa., Bacillus subtilis., Sarcina lutea., and four strains of Vibrio cholerae.. The bioactive compound 5,7,4′-trimethoxyflavone was identified.
Introduction
In Mexico, Tagetes lucida. Cav. (Tagetes florida. Sweet) is widely used in traditional medicine. Stems, leaves, and flowers are used against different kinds of ailments, such as dysentery, diarrhea, and cough (Viesca, Citation1976; Aguilar, Citation1994aCitation1994b, Aquino et al., Citation2002; Argueta & Cano, Citation1994; Argueta & Zoila, Citation1994). Tagetes lucida. Cav. (Asteraceae), known as pericón. is an erect perennial herbaceous plant that can reach 1 m in height. This herb has a sweet-smelling odor, resembling the aniseed aroma. It is widely distributed in the Valley of Mexico and ruderal zones (Rzedowski, Citation1979aCitation1979b).In traditional medicine, it is prepared as an infusion and applied orally (Argueta & Cano, Citation1994). The purpose of this work was to evaluate the antimicrobial activity of Tagetes lucida. and to isolate and identify the active compound.
Materials and Methods
Plant material
Tagetes lucida. Cav. (Tagetes florida. Sweet) was obtained in July 1997 from the Sonora Market, Mexico City, and was identified by Dra. Edith López Villafranco. A specimen (voucher no. 25559) was deposited at Izta Herbarium, FES Iztacala, UNAM.
Microorganism strains
Strains of Shigella boydii. (ATCC 8700), Staphylococcus aureus. (ATCC 12398), Escherichia coli. (ATCC 25922), Enterobacter agglomerans. (ATCC 27155), Salmonella typhi. (ATCC 19430), Vibrio cholerae. serotype No-01 (ATCC 35971), Vibrio cholerae. INDRE 206 (isolated from polluted water), Vibrio cholerae. isolated from a clinical case (these strains belong to the type 01, enterotoxine producers, Inaba serotype, biotype “El Tor”), Vibrio cholerae. CDC V12, Enterobacter aerogenes., Staphylococcus epidermidis., Bacillus subtilis., and Sarcina lutea. were obtained from the Department of Microbiology, Facultad de Estudios Superiores Cuautitlán, UNAM. Pseudomonas aeruginosa. and Candida albicans. were isolated from patients and were kindly provided by the Clinical Analysis Laboratory of FES-Iztacala, UNAM. Bacteria were grown on Mueller–Hinton broth (Bioxon 260-1, Estado de Mexico, Mexico). Candida albicans. was grown in Sabouraud broth (C-222400 Bioxon, Estado de Mexico, Mexico).
Extract preparation
Dried plant (1000 g of leaves, stems, and flowers) were macerated in 5 l of ethanol (96%) during 25 days. The ethanol extract was filtered and concentrated at reduced pressure to yield 61.4 g of residue. The residue was successively extracted with hexane, chloroform, ethyl acetate, acetone, and methanol. Extracts were concentrated to approximately 10 ml at 40°C under reduced pressure, and they were dried to constant mass at 43°C. After determining the yields, extracts were stored at 4°C until use. Yield extracts were as follows: hexane 6 g (0.60%), chloroform 30.4 g (3.04%), ethyl acetate 0.50 g (0.05%), acetone 1.2 g (0.12%), and methanol 23.2 g (2.33%).
Antimicrobial testing
Antimicrobial activity of the extracts was determined in the disk bioassays (2 mg/disk) in accordance with the diffusion method of Kirby-Baüer (Van der Berghe & Vlietnick, Citation1991). As a positive control, kanamycin disks (30 µg) and nystatin disks (30 µg) for C. albicans. were used; as negative controls, disks with the solvents used in the extract process and evaporated overnight were used (in the same manner as experimental disks). Each experiment was carried out in triplicate (a variance multifactorial analysis was fulfilled). During the purification process, determinations of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were evaluated according to the technique of Koneman (Citation1985).
Active compound isolation
For the separation of an active compound, column chromatography (silica gel mesh 70-230 Sigma 5-2509 St. Louis, MD, USA) and preparative thin-layer chromatography (silica gel Merck Kieselgel 60-5553, Düren, Germany) were used. The ethyl acetate extract (2.03 g) was chromatographed on silica gel column and eluted with hexane–chloroform–ethyl acetate (1.2:0.8:0.8). Thirteen fractions were obtained and evaluated with 1 mg/ml by the Koneman technique. Antimicrobial activity was observed in fractions 7–11. Joined fractions were newly chromatographed in a column and eluted with hexane–chloroform–ethyl acetate (1.2:0.8:1.0); seven fractions were obtained and evaluated. Activity was observed in fractions 3 and 4. To purify the active compound, preparative thin-layer plates were used (silicagel 60 F 254 µ). A mixture of hexane–chloroform–ethyl acetate (1.2:0.8:0.8) was used as mobile phase; 15 and 9 bands were obtained and tested from fractions 3 and 4, respectively. Activity was observed in the fifth band of fraction 3 and in the third band of fraction 4. Both bands presented an Rf of 0.25. The yield obtained of the active compound at the end of the process was 19.5 mg.
Structure identification
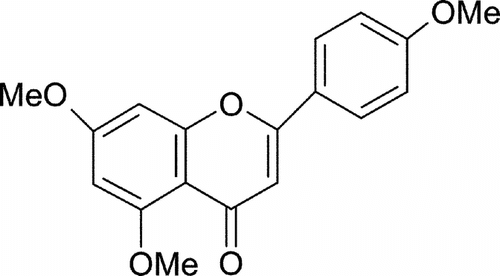
The active compound was identified by physical and spectroscopic studies: melting point 158–161°C. Infrared absorption (IR) (Perkin Elmer FT-IR Spectrum 2000), 2924.11 and 2852.91 cm−1 assignable to CH, –CH2 and CH3, 1717.06 cm−1 assignable to a ketone α, β-unsaturated; at 1615.41 cm−1 a sign that suggests the presence of an aromatic compound with alkyl groups substituted and a ketonic carbonyl. In 1H NMR (Varian Geminis 200 A, Palo Alto, CA, USA) δ ppm: 3.90, 3.92, 3.95, (9 H all s. 3 × OCH3); 6.32 (1 H, d., J. = 2.5 Hz, Ar H); 6.37 (1 H, d., J. = 2.5, Ar H); 6.67 (1 H, s., Ar COCH = C Ar); 6.86 (2 H, d., J. = 9.2 Hz, 2 × Ar H); 7.63 (2 H, d., J. = 9.2 Hz, 2 × Ar H). COSY spectra confirms the joining among the protons 2′ and 6′ with 3′ and 5′ forming a defined system A2B2, and correlation between 8 and 6 aromatic protons. 13C NMR presents chemical signs that were assigned to the carbons of the proposed structure following the pattern of Harborne (Citation1994) (C-2 163.0, C-3 103.627, C-4 174.4, C-5 158.0, C-6 99.949, C-7 162.0, C-8 107.885, C-9 153.0, C-10 103.261, C-1′ 120.0, C-2′ 128.025, C-3′ 115.145, C-4′ 161.0, C-5′ 115.145, C-6′ 128.025, methoxy groups 56.308 ppm). DEPT indicates the presence of 3 methyl/methoxy groups (–OCH3) and 7 methine groups (CH). Electron impact mass spectra (E.I.-MS) (Finnigan Mat GCQ Austin, TX, USA) showed that the molecular weight of the compound was 312. Data confirm that the compound responsible for antimicrobial activity is a methoxyflavone, the 5,7,4′-trimethoxyflavone (Jaipetch et al., Citation1983).
Results and Discussion
Antimicrobial assay
Antimicrobial susceptibility of each extract is shown in . Acetone and ethyl acetate extracts inhibited the growth of 10 bacterial strains: S. aureus., S. epidermidis., B. subtilis., S. lutea., Vibrio cholerae. (No-01), Vibrio cholerae. INDRE 206 (isolated from contaminated water), Vibrio cholerae. isolated from a clinical case, Vibrio cholerae. CDC V12, S. boydii., and P. aeuruginosa.. Methanol extract showed activity against S. boydii., S. epidermidis., Vibrio cholerae. INDRE 206 (isolated from polluted water), and Vibrio cholerae. CDC V12. Hexane extract only showed activity against S. aureus., S. epidermidis., and B. subtilis.. Chloroform extract was the only one that inhibited C. albicans. growth.
Table 1 Antimicrobial activity of the extracts from Tagetes lucida. (inhibition zone in millimeters).
Determination of MIC and MBC
Minimum inhibitory and minimum bactericidal concentrations of 5,7,4′-trimethoxyflavone were evaluated. Results are shown in . We can observe that V. cholerae. strains were more sensitive than other bacterial strains.
Table 2 Minimum inhibitory (MIC) and minimum bactericidal (MBC) concentration of 5,7,4′-trimethoxyflavone.
The ethyl acetate extract showed the largest inhibition zones. Variance analysis (p < 0.0008) was carried out to verify significant differences in susceptibility.
In accordance with our results, Gram-positive and Gram-negative bacteria were susceptible to the ethyl acetate and acetone extracts. However, Gram-negative species showed larger inhibition zones than Gram-positive species. C. albicans. showed susceptibility to the chloroform extract.
Because the ethyl acetate extract was active against bacteria, we proceeded to isolate the active compound. According to spectroscopic studies, the active compound was 5,7,4′-trimethoxyflavone (). Both physical and spectral parameters of this compound coincided with those reported by Jaipetch (Citation1983). Flavones have been reported to have different biological effects such as antibiotic activity (Harborne, Citation1994). This is in concordance with the activity observed in 5,7,4′-trimethoxyflavone against 11 bacterial strains and one yeast strain. It is understood that flavones can inhibit the synthesis of genetic material of bacteria and mediate antibiotic activity. V. cholerae. strains were more sensitive: MIC 0.75 mg/ml and MBC 1.0 mg/ml () than the other microorganisms.
Conclusions
The compound isolated, 5,7,4′-trimethoxyflavone (C18H16O5), from the ethyl acetate extract of Tagetes lucida. presented antimicrobial activity against Gram-positive and Gram-negative bacteria.
Acknowledgments
The authors are grateful to Dra. Edith López Villafranco, head of the Izta Herbarium, for her invaluable collaboration in plant identification and to Andres Martinez for technical assistance.
References
- Aguilar A, Camacho JR, Chino S, Jácquez P, López ME (1994a): Herbario Medicinal del Instituto Mexicano del Seguro Social. Información Etnobotánica. IMSS, México.
- Aguilar A, Camacho JR, Chino S, Jácquez P, López ME (1994b): Plantas Medicinales del Herbario IMSS. México, IMSS, pp. 148, 150.
- Aquino R, Caceres A, Morelli S, Rastrelli L (2002): An extract of Tagetes lucida. and its phenolic constituents as antioxidants. J Nat Prod. 65: 1773–1776, [PUBMED], [INFOTRIEVE], [CSA]
- Argueta VA, Cano AJ (1994): Atlas de las Plantas de la Medicina Tradicional Mexicana. México, Instituto Nacional Indigenista, pp. 15–34, 1141.
- Argueta A, Zolla C (1994): Nueva. Bibliografía de la Medicina Tradicional Mexicana. México, Instituto Nacional Indigenista.
- Harborne JB (1994): The Flavonoids: Advances in Research Since 1986. London, Chapman & Hall, pp. 259–330.
- Jaipetch T, Reutrakul V, Tuntiwachwuthkul P, Santisuk T (1983): Flavonoids in the black rhizomes of Boesenbergia pandurata.. Phytochemistrey 22: 625–626, [CROSSREF], [CSA]
- Koneman WE (1985): Diagnóstico Microbiológico. México, Médica Panamericana, pp. 380–402.
- Rzedowski GC (1979a): La Flora Fanerogámica del Valle de México. Editado por Rzedowski J. Vol I. México, D.F., Compañía Editorial Continental, pp. 144–147.
- Rzedowski GC (1979b): La Flora Fanerogámica del Valle de México. Editado por Rzedowski J. Vol II. México, D.F., Escuela Nacional de Ciencias Biológicas (ENCB), Instituto Politecnico Nacional (IPN), pp. 586–589.
- Van der Berghe DA, Vlietnick AJ (1991): Screening methods for antibacterial agents from higher plants. In: Hostettmann, K, ed., Methods in Plant Biochemistry “Assay for Bioactivity,” Vol 6. London, Academic Press, pp. 47–71.
- Viesca TC (1976): Estudios Sobre Etnobotánica y Antropología Médica. Ed. Instituto Mexicano para el Estudio de las Plantas Medicinales. Asociación Civil (A. C.), México.