Abstract
The major chemical components of Oman dill herb oil (oil from the leaves and flowering tops of Anethum graveolens. L. grown in Oman) were analyzed by 13C NMR spectroscopy. The activities of oil against bacteria, fungi, and aphids were also evaluated. The oil was inactive against Gram-positive and Gram-negative bacteria at low concentrations. It inhibited the growth of Candida albicans. and some molds that cause crop or food damage but demonstrated weak contact toxicity to aphids. The most abundant oil components were identified as limonene and dillapiole by 13C NMR analysis. Carvone, the spicy and antibacterial component of European dill oil, was absent.
Introduction
Anethum graveolens. L. syn. Peucedanum graveolens. Benth. et Hook, Apiaceae (Umbelliferae), trivially referred to as European dill, is indigenous to the Mediterranean and Caucasus countries. It grows naturally in the Dhofar region of Oman. It is an annual herb, about 50 cm tall and has striated, delicate, and hollow stems; pinnate leaves that are divided into segments; and white flowers that are borne in large umbrella-like ray of stems.
In Oman, the leaves of Anethum graveolens., or shabt., as it is locally called, are used to spice food and the seeds to flavor tea. The seeds are also boiled with water and drunk as a carminative medicine or to treat abdominal colic (Ghazanfar, Citation1992Citation1994). Anethum graveolens. is a high-oil-yielding plant: oil content of dill herb or weed varies from 0.10% to 0.3% (v/fresh weight) and dill seed from 1.8% to 4.0% (v/dry weight) (Badoc & Lamarti, Citation1991). The major components of European dill seed oil are carvone, dihydrocarvone, and limonene (Jin et al., Citation1996; Renata & Erwin, Citation2000; Kubeczka & Formacek, Citation2002; Jirovetz et al., Citation2003). European dill seed oil is sometimes used in place of caraway oil [= oil from the seed of Carum carvi. L. Apiaceae (Umbelliferae)] in perfumery because limonene and carvone constitute more than 90% of the total oil (Kubeczka & Formacek, Citation2002). However, dillapiole and isomeric mixtures of limonenes or phellandrenes have been detected as the major components of some dill herb oil (Huopalahti & Linko, Citation1983; Porter et al., Citation1983; Charles et al., Citation1995). Dill oil that demonstrates relatively strong antibacterial properties has limonene and carvone as major constituents (Jirovetz et al., Citation2003).
The aim of this investigation was to examine the major chemical components of Oman dill herb oil using NMR spectroscopy as a fingerprint and to evaluate the biological activity of oil against clinically important bacteria, pathogenic fungi, and aphids.
Materials and Methods
Plant material
Fresh tops and flowers of Anethum graveolens. were collected in the third week of September 2002 from Dhofar, Oman: approximately 4.4 km from Ma'amura Roundabout on Salalah-Marbat Road in a shallow, sandy depression on a coastal plain at an altitude of about 40 m. Dr. Shahina Ghazafar of the Royal Botanic Gardens (Kew, UK) identified the plant, and a specimen (voucher no. NP 018) is preserved in the University Herbarium, Department of Biological Science, Sultan Qaboos University, Oman.
Instruments
13C NMR spectra were recorded on a Bruker Avance (Switzerland) 400 MHz spectrophotometer operating at 100.58 MHz. Oil was dissolved C6D6 (4:1, v/v), with tetramethylsilane as an internal standard. The chemical shifts are reported in ppm. IR spectrum was taken on a Nicolet FT-IR (Madison, USA) spectrometer, optical rotation was measured with Perkin Elmer Model 341 Polarimeter (Norwalk, USA) in ethanol at 589 nm and 20°C, and the UV spectra was recorded on a UV-visible HP-8453 spectrophotometer (Germany).
Hydrodistillation
Fresh tops and flowers (500 g) of Anethum graveolens. and water (1:3) in a 10-quart stainless StoveStill apparatus (Essential Oil University, New Albany, IN, USA) were steam-distilled by heating on a hot plate, and 1.5 ml (0.36% v/wt) of a light yellow oil with a pleasant scent was obtained after 3 h of distillation.
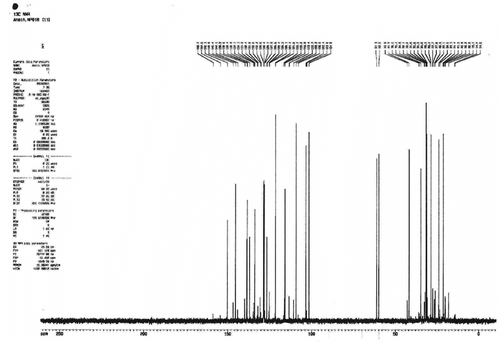
Light yellow oil: IR υmax cm−1 (neat): 3080, 2926, 1637, 1473, 1422, 1284, 1216, 1061, 915; UV λmax nm (ethanol): 242, 288, 335; 13C NMR (C6D6) data are shown in and . [α]D20° + 35.93° (c.0.014, ethanol).
Table 1 13C NMR of the oil.
Antimicrobial assays
Minimum inhibitory concentration (MIC) was determined according to NCCLS broth microdilution procedure (Anonymous, Citation2001) against Escherichia coli. (ATCC 9637), Klebsiella pneumoniae. (ATCC 27853), Pseudomonas aeruginosa. (ATCC 37853), Staphylococcus aureus. (ATCC 29213), Salmonella choleraesuis. (ATCC 14028), Bacillus subtilis. (clinical isolate), and Candida albicans. (ATCC 10231), using gentamicin sulfate and miconazole in dimethyl sulfoxide (DMSO) as positive controls. Aliquots (100 µL) of gradient concentrations in twofold dilutions were made in the wells of a microtiter plate from a stock of 1000 µg/ml of test sample in DMSO, using sterile nutrient broth as a diluent. Each well was inoculated with 10 µl of the test organism in culture (diluted to 0.5 McFarland standard with saline) and incubated at 37°C for 24 h to determine the minimum inhibitory concentration. The MIC values of oil and standards are shown in .
Table 2 Antimicrobial activity of oil.
Antifungal bioassay
A mixture of oil (150 mg) and 1 ml Tween 20 (0.01%) was added to molten potato dextrose agar medium (149 ml) to give a test solution of 1000 µg/ml. Lower concentrations of 250 and 125 µg/ml were similarly prepared. The antifungal activity of the oil at different concentrations was tested against Alternaria alternata. (Fr.) Keissler, Stemphylium solani. Weber, Curvularia lunata. (Wakker) Boedijn, Fusarium oxysporium. Schlecht, and Bipolaris. sp. using modified poisoned food technique (Grover & Moore, Citation1962; Janssen et al., Citation1987) and as reported in our previous publication (Al-Burtamani et al., Citation2005). The fungitoxicity of oil was recorded in terms of mycelial inhibition using the expression:
where MIc and MIt are the average diameters of mycelial colonies in control and treated fungi sets, respectively. The antifungal activities are summarized in .
Table 3 Antifungal activity of oil against phytopathogenic fungi.
Toxicity test with aphids
Leaves of cucumber plants that were infected or free of Aphis gossypii. were excised from plants at Al-Hail and Al-Amrat local farms and transferred into the laboratory in plastic bags. Fifty apterous adult aphids were placed on fresh aphid-free leaves. The petioles of the leaves were held in water-soaked cotton plugs. The aphids were allowed to settle on the leaves for 1 h. Test solutions (100 µL) at 50, 25, 12.5, and 6.25 µg/ml and of 0.01% aqueous Tween 20 and of a solution of Permasect (a commercial insecticide used against insects in the field at 150–200 ml in 100 l of water), 2 ml per liter of water, was separately applied to each colony of 50 aphids using a micropipette. Each treatment was duplicated, and the survivors were counted at 12-h intervals for 24 h in the test and control experiments. Where the number of dead aphids in the blank control was two or less, the percentage mortality was corrected using Abbott's formula. The percentage mortality was estimated using the expression:
where SE is the number of aphids that survived in test sample, and SC is the number that survived in the blank control.
The LC50 of oil on aphids was computed from the results at 95% confidence interval using the Finney program, and the results are shown in .
Table 4 Mortality of Aphis gossypii. after topical application of Anethum. oil.
Results and Discussion
Dill herb oil has a very pleasant aroma. The major components of the Oman dill herb oil were found to be limonene and dillapiole from NMR analysis (see and ). The 13C NMR spectra of oil lacked signals at δC 197.43 (carbonyl signal), 147.21, 143.27, 135.68, 110.43, 43.21, 42.79, 31.37, 20.25, and 15.58 that are characteristic of carvone (Kubeczka & Formacek, Citation2002).
Oman dill oil has optical rotation of + 35.93°. European dill oil has optical rotation in the range of + 70° to + 80°, and optical rotation between + 41° and + 47° has been reported for India dill oil (Anonymous, 1911). In this respect, Oman dill oil is very similar to Indian showa. dill oil.
The antimicrobial assay showed that Oman dill oil selectively inhibited the growth of Candida albicans. and Escherichia coli. at MIC values of 32 and 250 µg/ml, respectively (). Although the level of inhibition of Candida albicans. by oil is about 15-times less potent than miconazole (), the oil demonstrated selective antimicrobial activity against some gut microbes. This could confer some health benefits on communities that use dill herb as medicine.
In addition, limonene and dillapiole have previously been demonstrated in mouse target tissues and in vitro. tests to be potentially cancer chemopreventive (Zheng et al., Citation1992; Teissedre & Waterhouse, Citation2000). The oil was, however, ineffective against Klebsiella pneumoniae, Pseudomonas aeruginosa., Staphylococcus aureus., Salmonella choleraesuis., and Bacillus subtilis..
In the antifungal assay, the oil generally showed weak activity but inhibited the growth of Stemphylium solani. Weber, Curvularia lunata. (Wakker) Boedijn, Fusarium oxysporium. (Schlecht) and Bipolaris. sp. by more than 70% after 4 days of single treatment at 250 µg/ml (). Monoterpene hydrocarbons have been demonstrated to inhibit the mycelia growth of Curvularia pallescens. and Fusarium oxysporium. (Singh et al., Citation2002) and the growth of a variety of molds that cause crop damage or food spoilage (Aggarwal, Citation2002).
When tested against Aphis gossypii. Glover (Homoptera; Aphididae), the oil was apparently ineffective, demonstrating weak lethality (). All aphids treated with permasect insecticide at 0.02% aqueous solution died within 25 minutes of treatment. Aphis gossypii. and Brevicoryne brassicae. Linn. (Homoptera: Aphididae) are commonly sighted on cucumber, cabbage, radish, and cauliflower crops in Oman. They cause direct damage to the photosynthetic apparatus of plants as a result of feeding activity, resulting in stunted plant growth.
Limonene and dillapiole are the most abundant chemical components of Oman dill oil as revealed by 13C NMR analysis. This technique could serve as a convenient method for monitoring seasonal changes in the composition of dill herb oil. Oman dill oil will better serve as a fragrant antifungal agent when used on food; its lethality to aphids is rather weak to be useful as an insecticide.
Acknowledgment
This project was funded by grants SR/SCI/CHEM/01/01 at Sultan Qaboos University.
References
- Aggarwal KK, Khanuja SPS, Ahmad A, Kumar TRS, Gupta VK, Kumar S (2002): Antimicrobial activity profiles of the two enantiomers of limonene and carvone isolated from the oils of Mentha spicata and Anethum sowa., Flavour Fragr J 17: 59–63.
- Anonymous (2001): Methods for antimicrobial susceptibility testing of anaerobic bacteria; Approved standard M11-A5; 5th ed. In: National Committee for Clinical Laboratory Standards, Vol. 21. Wayne, PA, NCCLS, p. 14.
- Anonymous (1911): Oil of dill. In: Oleum Anethi, BP, ed., British Pharmaceutical Codex. Published by direction of the Council of the Pharmaceutical Society of Great Britain.
- Al-Burtamani SKS, Fatope MO, Marwah RG, Onifade AK, Al-Saidi SH (2005): Chemical composition, antibacterial and antifungal activities of the essential oil of Haplophyllum tuberculatum. from Oman. J Ethnopharmacol 72: 107–112, [CROSSREF]
- Badoc A, Lamarti A (1991): A chemotaxonomic evaluation of Anethum graveolens. L. (Dill) of various origins. J Essential Oil Res 3: 269–278.
- Charles DJ, Simon JE, Widriechner MP (1995). Characterization of essential oil of dill (Anethum graveolens. L.). J Essential Oil Res 7: 11–20.
- Ghazanfer SA (1992): An Annotated Catalogue of the Vascular Plants of Oman, and their Vernacular Names. Scripta Botanica Belgica 2. Meise: National Botanic Garden of Belgium.
- Ghazanfar SA (1994): CRC Handbook of Arabia Medicinal Plants. Boca Raton, FL, CRC Press.
- Grover RK, Moore JD (1962): Toximetric studies of fungicides against the brown rot organisms, Sclerotinia fruticola. and S. laxa.. J Phytopath 52: 876–880.
- Huopalahti R, Linko RR (1983): Composition and content of aroma compounds in dill, Anethum graveolens. L., at three different growth stages. J Agric Food Chem 31: 331–333, [CROSSREF]
- Janssen AM, Scheffer JJ, Baerheim SA (1987): Antimicrobial activity of essential oils: A literature review (1976–1986). Aspects of the test method. Planta Medica 53: 395–398, [PUBMED], [INFOTRIEVE]
- Jin Y, Shi C, Wang Y, Xu H (1996): Chemical constituents of dill seed oil, the essential oil of Anethum graveolens. L. Zhongcaoyao 27: 654.
- Jirovetz L, Buchbauer G, Stoyanova AS, Georgiev EV, Damianova ST (2003): Composition, quality control, and antimicrobial activity of the essential oil of long-time stored dill (Anethum graveolens. L.) seeds from Bulgaria. J Agric Food Chem 51: 3854–3857, [PUBMED], [INFOTRIEVE]
- Kubeczka KH, Formacek V (2002): Essential Oils Analysis by Capillary Gas Chromatography Carbon-13 NMR Spectroscopy, 2nd ed. West Sussex, England, John Wiley & Sons.
- Porter NG, Shaw ML, Shaw GJ, Ellingham PJ (1983): Content and composition of dill herb oil in the whole plant and different plant parts during crop development. New Zealand. J Agric Res 26: 119–127.
- Renata Z-W, Erwin W (2000): Enantiomeric composition of limonene and carvone in seeds of dill and caraway grown in Poland. Polish J Food Nutr Sci 9: 9–13.
- Singh G, Singh OP, Maurya S (2002): Chemical and biocidal investigations on essential oils of some Indian Curcuma. species. Prog Cryst Growth Charact Mater 45: 75–81, [CROSSREF]
- Teissedre PL, Waterhouse AL (2000): Inhibition of oxidation of human low-density lipoproteins by phenolic substances in different essential oils varieties. J Agric Food Chem 48: 3801–3805, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Zheng GQ, Kenney PM, Lam LKT (1992): Anethofuran, carvone, and limonene: Potential cancer chemopreventive agents from dill weed oil and caraway oil. Planta Medica 58: 338–341, [PUBMED], [INFOTRIEVE]