Abstract
The current study was designed to evaluate whether compounds isolated from local medicinal plants in Malaysia suppressed nitric oxide (NO) production in inflammation. The murine monocytic macrophage (RAW 264.7) cell line was used as a target cell and activated by interferon-γ. (IFN-γ.) and lipopolysaccharide (LPS). Our current study has identified four phytochemicals, namely atrovirinone, cardamonin, flavokawin B, and zerumbone, that inhibit pathological NO generation. These compounds are candidates for further bioassay studies to determine their suitability as drug leads.
Keywords:
Introduction
Nitric oxide (NO) is a major secretory product of mammalian cells that initiates host defense, homeostatic, and development functions by either direct or indirect effects (Matthew et al., Citation1999). Direct effects are those reactions in which NO interacts directly with a biological molecule or target, whereas indirect effects are those reactions mediated by NO-derived intermediates such as peroxynitrite (ONOO−) derived from the reaction of NO with superoxide (O2−). The direct effects occur at lower concentrations or fluxes of NO, whereas higher NO fluxes result in indirect effects. Under normal physiological conditions, cells containing constitutive isoforms of inducible nitric oxide synthase (NOS) (endothelial or neuronal NOS) produce relatively small but significant amounts of NO, which will predominate in the normal tissue. However, as in the case of chronic inflammation, the high output of NO is approximately>10μ.M (Burgner et al., Citation1999), the inducible isoform of NOS (iNOS) is upregulated, and the reaction of formation of highly toxic compounds will occur such as nitrosation, nitration, and oxidation.
It has been reported that excess production of NO by macrophages is thought to promote a number of chronic inflammatory diseases such as arthritis, hepatitis, septic and hemorrhagic shock, and certain autoimmune disorders (Onur et al., Citation2001; Boveris et al., Citation2002; McDonald et al., Citation2003; Koulentaki et al., Citation2004; Zehntner et al., Citation2004). Increased expression of iNOS and/or its catalytic activity had been discovered in human tumor tissues (Wang et al., Citation2003; Franco et al., Citation2004; Loibl et al., Citation2005). The pathological effects of NO are due to reactive nitrogen oxide species formation in the presence of molecular oxygen (O2), which can damage DNA, inhibit a variety of enzymes, and initiate lipid peroxidation. Therefore, NO is a pivotal chemical indicator of inflammation and inflammatory disease.
The generation of NO from L-arginine proceeds via the formation of N-hydroxy-L-arginine (Pufahl et al., Citation1992). This L-arginine or NO pathway can be inhibited by several analogues of L-arginine such as Nω-monomethyl-L-arginine (L-NMMA), Nω-nitro-L-arginine (L-NA), and Nω-nitro-L-arginine methyl ester (L-NAME), which are nonselective NOS inhibitors (Moncada & Higgs, Citation1993). Other report has shown that consumption of these NOS inhibitors can result in accumulation in human plasma and urine, which could contribute to the pathophysiology of some conditions (Vallance et al., Citation1992). Some of the selective iNOS inhibitors are N-δiminoethyl-L-ornithine (L-NIO) and aminoguanidine. Many scientists continue to search for a selective inhibitor of iNOS in their attempts to develop more effective and less toxic drugs. Thus far, the drugs available affect both the inducible and the constitutive isoforms.
Recently, several iNOS inhibitors were reported from plants such as chalcones (Koorbanally et al., Citation2003), benzoquinone (Niwa et al., Citation1997), sesquiterpene lactones (Matsuda et al., Citation1999), and zerumbone (Murakami et al., Citation2002). Most of these compounds showed their inhibitory activity of NO production through the inhibition of iNOS expression. Today, much effort has been focused on the plants for potentially useful products as commercial iNOS inhibitors or lead compounds for drug development. Although pharmacological and epidemiological investigations have demonstrated that medicinal plants have anticancer, antimicrobial, and antiviral activities, little work has been done to find iNOS inhibitors.
In order to search for potentially new safer types of anti-inflammatory agents as lead compounds, we have screened various naturally occurring phytochemicals isolated from Malaysian medicinal plants on the multifunctional signaling molecule of NO in the murine monocytic macrophage (RAW 264.7) cell line after stimulation with IFN-γ./LPS.
Materials and Methods
Reagents
Fetal calf serum was purchased from Mycoplex (PAA Lab. GmbH, Linz, Austria). Antibiotic 5000 U/mlpenicillin/5000 μ.g/ml streptomycin and DMEM without phenol red were purchased from Flowlab (Flowlab, Sydney, Australia). Recombinant mouse IFN-γ. was purchased from BD Pharmingen (San Diego, CA, USA). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, FW 414.32) was purchased from Fluka, BioChemika (Buchs, Switzerland). Dimethyl sulfoxide (FW 78.13) was purchased from BDH Laboratory Supplies (Poole, England). Escherichia coli. lipopolysaccharide with serotype 055:B5, trypan blue (MW 960.8), and Nω.-nitro-L-arginine methyl ester (L-NAME) hydrochloride (MW 269.7) were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Test materials
All the pure compounds isolated from local plants were collected from the Laboratory of Natural Products, Institute of Bioscience, Universiti Putra Malaysia. The voucher specimens of plants have been deposited in this institute. In this screening test, all compounds were prepared at high concentration of 50 mM and dissolved in 100% DMSO and then serially diluted in media with 5% serum and added to the assays described below. Controls treated with vehicle (0.1%/dimethyl sulfoxide) were always run in parallel.
Cell culture
Murine monocytic macrophage (RAW 264.7) cell lines purchased from European Cell Culture Collection (CAMR, Porton Down, Salisbury, UK) were maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal calf serum (FCS), 4.5 g/l glucose, 1 mM sodium pyruvate, 2 mM L-glutamine, 50 µg/ml streptomycin, and 50 U/ml penicillin. Cells were grown at 37°C, 5% CO2 in fully humidified air and were split twice a week.
Cell stimulation
Cells with a confluency of 80–90% were scraped out and centrifuged at 110 × g/4°C for 10 min. The cell viability and concentration was determined simultaneously using trypan blue dye exclusion and a hemacytometer. Viability was always > 95%. The cell concentration was adjusted to 1 × 106 cells/ml. Then, 50 µl (5 × 104 cells/well) of cell suspension was dispensed into all wells of a tissue culture grade 96-well plate except for blanks and incubated for 2 h at 37 °C, 5% CO2 to allow cell attachment. After 2 h, unattached cells were gently discarded. Attached cells were then induced with 200 U/mlof recombinant mouse IFN-γ. (BD Pharmingen, San Diego, CA, USA) plus 10 µg/ml of E. coli. LPS (serotype 055:B5) in the presence or absence of test compounds at a final volume of 100 µl. Stock compounds (50 mM) were dissolved in 100% DMSO and serially diluted at a decreasing concentration of 12.5–50 µM. The final concentration of DMSO was 0.1% (v/v). This DMSO percentage allows the optimal solubilization of pure compounds in aqueous solution without toxic effect upon the cells. Controls with IFN-γ. and LPS also received the same amount of DMSO. Cells were then incubated at 37 °C, 5% CO2 for 17–20 h.
Measurement of nitric oxide formation
To evaluate the inhibitory activity of test materials on NO production, culture media was assayed for NO2− by the Griess reaction (Dirsch et al., Citation1988). Briefly, an equal volume of Griess reagent [1% sulfanilamide/0.1% N-(1-naphtyl)ethylene diamine dihydrochloride in 2.5% H3PO4] was mixed with cell culture supernatants, and color development was assessed at λ 550 nm with a microplate reader (SpectraMax, Plus 384, Molecular Devices, Inc., Sunnyvale, CA, USA). DMEM media only was measured as the blank in all the experiments. The amount of nitrite in the samples was calculated from a sodium nitrite standard curve freshly prepared in deionized water. The optical densities (OD) of test compounds were compared with the OD of the controls containing cells stimulated with IFN-γ./LPS plus 0.1% DMSO to assess the percent inhibition of nitrite (NO2−). The cells remaining in the tissue culture plates were then assayed for cell viability (MTT assay). Percent inhibition was calculated as
MTT assay for cell viability
Mitochondrial respiration, an indicator of cell viability, was assessed by the mitochondria-dependent reduction of MTT to formazan. Cells were incubated with MTT for 4 h at 37 °C. Briefly, 100 µl of DMEM containing 5% FCS was added to each well plus 20 µl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; 5 mg/ml in PBS). After 4 h, cell medium was removed, and the formazan crystal formation was dissolved with 100 µl/well of 100% DMSO. The extent of reduction of MTT to formazan within cells was quantified by measuring OD570 using a microplate reader. The optical densities (OD570) for the cell viability of the test compounds were compared with the OD of the controls containing cells with IFN-γ./LPS plus 0.1% DMSO to assess the viability. Percent of cell viability was calculated as
Results
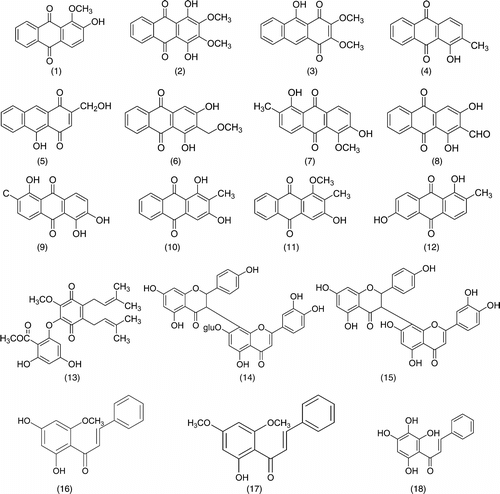
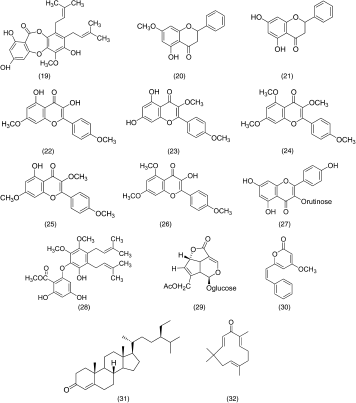
A total of 32 pure compounds were screened for their inhibitory effect on NO release in RAW 264.7 cells. Pure compounds were considered as having a strong, moderate, or weak activity if inhibition of NO release was more than 90%, between 50% and 90%, or less than 50%. From the 32 phytochemicals (Scheme ), only two compounds, cardamonin (16) and zerumbone (32), exhibited higher than 90% inhibition of NO production at the highest concentration of 50 µM. However, 50 µM of zerumbone was associated with a small degree of cytotoxicity. Coincubation of macrophages in the presence of test compounds at 12.5, 25, and 50 µM and IFN-γ./LPS resulted in a dose-dependent reduction of NO production, especially, the pure compound of flavokawin B (17) and atrovirinone (13), which showed higher than 50% inhibition at the concentration of 25 µM without any cytotoxicity. Other compounds showed either a moderate or weak inhibitory activity at the highest concentration tested. At 50 µM, many compounds showed higher than 100% inhibitory effects on NO production but their cytotoxic effect was high. The overall efficacy of all compounds on nitrite production in IFN-γ./LPS–activated macrophage is shown in . The generation of NO from L-arginine proceeds via the formation of Nω.-hydroxy-L-arginine (Pufahl et al., Citation1992). This L-arginine/NO pathway can be inhibited by several analogues of L-arginine such as Nω.-nitro-L-arginine methyl ester (L-NAME), a nitric oxide synthase (NOS) inhibitor. In our preliminary study, L-NAME was run parallel as a positive control (data not shown).
Table 1 The effect of 32 pure compounds isolated from Malaysian plants on NO production and cell viability of RAW 264.7 macrophage cells.Footnotea.
Discussion
A variety of cells such as macrophages are able to synthesize large amounts of nitric oxide (NO) from L-arginine in a reaction catalyzed by NO synthase (NOS) (Onur et al., Citation2001). Quiescent macrophages can be stimulated with interferon-γ. (IFN-γ.) and lipopolysaccharide (LPS) to activate inducible nitric oxide synthase (iNOS), after which is produced large amounts of NO (Bogdan et al., Citation2000). Overproduction of NO in the immune system acts as a major cytotoxic mediator and inhibits the growth of invading microorganisms and tumor cells. However, the excessive production of NO will interact with superoxide anion (O−) and forms highly reactive oxidant peroxynitrite anions (ONOO−), which in turn induce inflammatory cytokines, which leads to cytokine-induced cell death by apoptosis and necrosis (Burgner et al., Citation1999).
Several studies have revealed the increased expression of iNOS and/or its catalytic activity in asthma (Kharitonov et al., Citation1994), rheumatoid arthritis (RA) (Onur et al., Citation2001) and human tumor tissues (Goldstein et al., Citation1998). Thus, there are many efforts to develop enzyme inhibitors or repressors of enzyme formation that are selective for the inducible forms of these enzymes and do not affect the desirable activity of their respective constitutive isoforms. Indeed, selective iNOS inhibitor, such as aminoguanidine, has been reported to exert therapeutic effects by preventing asthma and infarction (Richardt et al., Citation2005; Willmota et al., Citation2005). We attempted to screen several compounds from Malaysian medicinal plants, which have not yet been scientifically evaluated for NO inhibitory activity. This preliminary study was aimed at investigating the anti-inflammatory activities of 32 pure compounds isolated from different species of indigenous plants, which were classified into several structural groups, namely, chalcones, flavones, flavanones, flavonol glycoside, biflavonoids, depsidone, hydroquinone, benzoquinone, anthraquinones, kavapyrone, sesquiterpene, sterol, and iridoid glucoside. Thus, the activity exhibited by these compounds may justify the medicinal claims and usefulness of the respective species in which the active compounds are present. These compounds may also serve as leads that could be further assessed for anti-inflammatory activity.
Among the four active compounds, two of them, cardamonin (16) and flavokawin B (17), are chalcone derivatives (). Our results indicated that the presence of one methylated OH (at the C-2′ position in ring A) or two methylated OH (C-2′ and C-4′ in ring A) of these trihydroxylated chalcones might attenuate their inhibitory effects on NO production. Other chalcone such as 4-dimethylamino-3′,4′-dimethoxychalcone has been described as having significant anti-inflammatory activity, though inhibition of iNOS protein expression might be due to the presence of methoxy groups on its benzoyl ring (Herencia et al., Citation2001). This is in contrast with 2′,3′,4′,6′-tetrahydroxychalcone (18), which was found inactive although it showed very significant activity as compared with cardamonin and flavokawin B on a radical scavenging assay (Habsah et al., Citation2004). Based on these observations, we believed that hydroxylation of the ring A in chalcones increases their radical scavenging potential. However, their presence reduces the NO production inhibition possibly due to the decreases in lipophilicity of the benzoyl end of the chalcones (ring A). Methylation of one or more of the hydroxyl groups increases the lipophilicity and, thus, increases its potential to penetrate the cell wall to affect NO inhibition. Other flavonoid derivatives, including flavones, flavanones, flavonol glycoside, and biflavonoids, tested in this study did not show a significant effect on the NO production.
Zerumbone (32), a major monocyclic sesquiterpene ketone isolated from the rhizomes of Zingiber zerumbet. Smith, also showed a high degree of NO production inhibition. This result is consistent with reports by Murakami et al. (Citation2002), who suggested that zerumbone significantly inhibited NO production by suppressing inducible nitric oxide synthase expression (iNOS) through the attenuation of transcription factor of nuclear factor κB (NFκB) (Murakami et al., Citation2003). They also suggested that the α,β.-unsaturated carbonyl group moiety was important for zerumbone anti-inflammatory effects (Murakami et al., Citation1999).
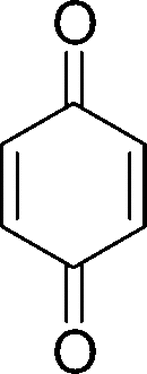
Synthetic quinone-containing compounds have been used for therapeutic purposes, including the treatment of cancer and inflammation, and their biological effects have been extensively studied. In this study, three subgroups, hydroquinone, benzoquinone, and anthraquinones, were tested. Among these compounds, only atrovirinone was active as a NO production inhibitor. Atrovirinone (13), a benzoquinone derivative isolated from the roots of Garcinia atroviridis. (Permana et al., Citation2001), strongly inhibited the production of NO with low cytotoxicity at a concentration of 12.5 µM. Niwa et al. (Citation1997) have shown that benzoquinone () inhibited the production of NO induced by IFN-γ./LPS in rat C6-glia cells through the suppression of iNOS mRNA expression. A related study on prenylated flavonoids has shown that chemical entities having an isoprenyl (3,3-dimethylallyl), a geranyl (E.-3,7-dimethyl-2,6-octadienyl), a 1,1-dimethylallyl, and/or a lavandulyl (5-methyl-2-isoprophenyl-hex-4-enyl) moiety as part of their backbone structure are usually more hydrophobic than the conventional chemicals, suggesting easy penetration through the cell membrane so that they can react with the cyclooxygenase and lipoxygenase enzymes (Chi et al., Citation2001). Consistent with this observation, we suggested that the prenylated benzoquinone skeleton in atrovirinone might be important for the inhibitory activity of NO production. Other quinones tested in this study showed either no effect on NO production or cytotoxicity toward macrophage cells. In contrast to the above observation, among the 12 anthraquinones studied, 1,4-dihydroxy-2,3-dimethoxylanthraquinone and morindone showed an enhancement rather than an inhibitory effect on NO production. It is interesting to note that although many quinones have been found to exhibit cytotoxic effects through the generation of reactive oxygen species via enzymatic redox cycle or the loss of protein thiols caused by arylation (Chung et al., Citation1997), atrovirinone, 1,4-dihydroxy-2,3-dimethoxylanthraquinone, and morindone did not show cytotoxic effects except at the highest concentration of 50 µM. However, atrovirinone at the lower concentration of 12.5 µM, had strong inhibitory activity on NO production without any cytotoxic effects.
In conclusion, we have identified four phytochemicals, namely, atrovirinone, cardamonin, flavokawin B, and zerumbone, as having potential therapeutic value for the treatment or prevention of certain acute or chronic inflammatory diseases associated with pathological NO generation. All four compounds may have potential as lead compounds for the development of more potent drugs to inhibit NO production in macrophages. Thus, further bioassay studies on the mechanism of action are proposed in order to establish the practical value of these compounds.
Acknowledgment
This work was supported by IRPA grant number 09–02–04–0274-EA001 from the Ministry of Science, Technology and Innovation, Malaysia.
References
- Bogdan C, Röllinghoff M, Diefenbach A (2000): Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Current Opinion in Immunology 12: 64–76, [PUBMED], [INFOTRIEVE]
- Boveris A, Alvarez S, Navarro A (2002): The role of mitochondrial nitric oxide synthase in inflammation and septic shock. Free Radical Biology & Medicine 33: 1186–1193, [CROSSREF]
- Burgner D, Rockett K, Kwiatkowski D (1999): Nitric oxide and infectious diseases. Archives of Disease in Childhood 81: 185–188, [PUBMED], [INFOTRIEVE]
- Chi YS, Jong HG, Son KH, Chang HW, Kang SS, Kim HP (2001): Effects of naturally occurring prenylated flavonoids on enzymes metabolizing arachidonic acid: Cyclooxygenases and lipoxygenases. Biochemical Pharmacology 62: 1185–1191, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Chung JH, Seo DC, Chung SH, Lee JY, Seung SA (1997): Metabolism and cytotoxicity of menadione and its metabolite in rat platelets. Toxicology and Applied Pharmacology 142: 378–385, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Dirsh VM, Stuppner H, Vollmar AM (1998): The Griess assay: Suitable for a bio-guided fractionation of anti-inflammatory plant extracts? Planta Medica 64: 423–426.
- Franco L, Doria D, Bertazzoni E, Benini A, Bassi C (2004): Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in pancreatic cancer. Prostaglandins 73: 51–58.
- Goldstein SR, Yang GY, Chen X, Curtis SK, Yang CS (1998): Studies of iron deposits, inducible nitric oxide synthase and nitrotyrosine in a rat for esophageal adenocarcinoma. Carcinogenesis 19: 1445–1449, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Habsah M, Faridah A, Permana D, Lajis NH, Ali AM, Sukari MA, Hin TYY, Kikuzaki H, Nakatani N (2004): DPPH free radical scavenger components from the fruits of Alpinia rafflesiana. Wall. ex. Bak. (Zingiberaceae). Z. Naturforsch 59c: 811–815.
- Herencia F, Ferrándiz ML, Ubeda A, Guillén I, Dominguez JN, Charris JE, Lobo GM, Alcaraz MJ (2001): 4-Dimethylamino 3′,4′-dimethoxychalcone downregulates iNOS expression and exerts anti-inflammatory effects. Free Radical Biology & Medicine 30: 43–50, [CROSSREF]
- Kharitonov SA, Yates D, Robbins RA, Logan-Sinclair R, Shinebourne EA, Barns PJ (1994): Increased nitric oxide in exhaled air of asthmatics. Lancet 343: 133–135, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Koorbanally NA, Randrianarivelojosia M, Mulholland DA, van Ufford LQ, van den Berg AJJ (2003): Chalcones from seed of Cedrelopsis grevei. (Ptaeroxylaceae). Phytochemistry 62: 1225–1229, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Koulentaki M, Notas G, Petinaki E, Valatas V, Mouzas IA, Castanas E, Kouroumalis EA (2004): Nitric oxide and pro-inflammatory cytokines in acute hepatitis B. European Journal of International Medicine 15: 35–38, [CROSSREF]
- Loibl S, Buck A, Strank C, von Minckwitz G, Roller M, Sinn H-P, Schini-Kerth V, Solbach C, Strebhardt K, Kaufmann M (2005): The role of early expression of inducible nitric oxide synthase in human breast cancer. European Journal of Cancer 41: 265–271, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Matsuda H, Shimoda H, Uemura T, Yoshikawa M (1999): Preventive effect of sosquiterpenes from bay leaf on blood ethanol elevation in ethanol-loaded rat: Structure requirement and suppression of gastric emptying. Bioorganic & Medicinal Chemistry Letters 9: 2647–2652, [CROSSREF]
- Matthew B, Grisham, Jourd'Heuil D, Wink DA (1999): Nitric oxide. I. Physiological chemistry of nitric oxide and its metabolites: Implications in inflammation. American Journal of Physiology 276: G315–G321.
- McDonald M, Abdelrahman M, Cuzzocrea S, Thiemermann C (2003): Tyrphostin reduces the organ injury in haemorrhagic shock: Role of inducible nitric oxide synthase. Resuscitation 58: 349–361, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Moncada S, Higgs EA (1993): The L-arginine-nitric oxide pathway. The New England Journal of Medicine 329: 2002–2012, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Murakami A, Takahashi M, Jiwajinda S, Koshimizu K, Ohigashi H (1999): Identification of zerumbone in Zingiber zerumbet. Smith as a potent inhibitor of12-O.-tetradecanoylphorbol-13-acetate-induced Epstein-Barr virus activation. Bioscience, Biotechnology, and Biochemistry 63: 1811–1812, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Murakami A, Takahashi D, Kinoshita T, Koshimizu K, Kim HW, Yoshihiro A, Nakamura Y, Jiwajinda S, Terao J, Ohigashi H (2002): Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: The α,β.–unsaturated carbonyl group is a prerequisite. Carcinogenesis 23: 795–802, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Murakami A, Matsumoto K, Koshimizu K, Ohigashi H (2003): Effects of selected food factors with chemopreventive properties on combined lipopolysaccharide- and interferon-γ.-induced Iκ.B degradation in RAW264.7 macrophages. Cancer Letter 195: 17–25, [CROSSREF]
- Niwa M, Nakamura N, Kitajima K, Ueda M, Tsutsumishita Y, Futaki S, Takaishi Y (1997): Benzoquinone inhibit the expression of inducible nitric oxide synthase gene. Biochemical and Biophysical Research Communications 239: 367–371, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Onur Ö, Akinci AS, Akbiyik F, Ünsal I (2001): Elevated levels of nitrate in rheumatoid arthritis. Rheumatology International 20: 154–158, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Permana D, Lajis NH, Mackeen MM, Ali AM, Aimi N, Kitajima M, Takayama H (2001): Isolation and bioactivities of constituents of the roots of Garcinia atroviridis. Journal of Natural Products 64: 976–979, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Pufahl RA, Nanjappan PG, Woodard RW, Marletta MA (1992): Mechanistic probes of N-hydroxylation of L-arginine by the inducible nitric oxide synthase from murine macrophages. Biochemistry 31: 6822–6828, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Richardt G, Landgraf, Russo M, Jancar S (2005): Acute inhibition of inducible nitric oxide synthase but not its absence suppresses asthma-like responses. European Journal of Pharmacology 518: 212–220, [CROSSREF]
- Vallance P, Leone A, Calver A, Collier J, Moncada S (1992): Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339: 572–575, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Wang J, Torbenson M, Wang Q, Ro JY, Becich M (2003): Expression of inducible nitric oxide synthase in paired neoplastic and non-neoplastic primary prostate cell cultures and prostatectomy specimen. Urologic Oncology 21: 117–122, [PUBMED], [INFOTRIEVE]
- Willmota M, Gibsona C, Graya L, Murphya S, Philip B (2005): Nitric oxide synthase inhibitors in experimental ischemic stroke and their effects on infarct size and cerebral blood flow: A systematic review. Free Radical Biology & Medicine 39: 412–425, [CROSSREF]
- Zehntner SP, Bourbonniere L, Hassan-Zahraee M, Tran E, Owens T (2004): Bone marrow-derived versus parenchymal sources of inducible nitric oxide synthase in experimental autoimmune encephalomyelitis. Journal of Neuroimmunology 150: 70–79, [PUBMED], [INFOTRIEVE], [CROSSREF]