ABSTRACT
Orthosiphon stamineus. Benth. was cultivated at different locations in Malaysia to compare the xanthine oxidase inhibitory potential of the methanol extracts of the leaf. The extracts showed superoxide scavenging and xanthine oxidase inhibitory activities using in vitro. methods. By means of HPTLC densitometric technique and UV-Vis spectra, sinensetin (SEN) and 3′-hydroxy-5,6,7,4′-tetramethoxyflavone (TMF) concentrations in the extracts were found to vary from 0.09% to 0.36% and 0.02% to 0.10% of the day weight, respectively. The study provided evidence that the leaf of the plant can be useful for studying its anti-gout action.
Introduction
Orthosiphon stamineus. Benth. (Lamiaceae) contains several chemically active constituents such as terpenoids (diterpenes and triterpenes), polyphenols (lipophilic flavonoids and phenolic acids), and sterols (Tezuka et al., Citation2000). In Southeast Asia, the tea prepared from leaves of Orthosiphon stamineus. (OS) is taken as beverage to improve health and for the treatment of kidney and bladder inflammation, gout, and diabetes (Hegnauer, Citation1966; Wagner, Citation1982).The therapeutic effects of OS have been ascribed mainly to its polyphenols, which have enzyme inhibiting and antioxidant activities. Lipophillic flavonoids isolated from OS have been reported to show radical-scavenging activity toward the diphenylpicrylhydrazyl radical and inhibition of 15-lipoxygenase from soybeans used as model for mammalian 15-lipoxygenase (Lyckander & Malterud, Citation1996). Research indicates that the isolated flavones sinensetin (SEN) and 3′-hydroxy-5,6,7,4′-tetramethoxyflavone (TMF) from OS exhibited a diuretic activity in rats after intravenous administration, and the diuretic effect of the extracts could be partially due to its lipophilic flavones content (Schut & Zwaving, Citation1993).
Xanthine oxidase (XO) is considered to be an important biological source of superoxide radicals. Some superoxide radicals are capable of initiating lipid peroxidation by abstracting allylic protons from polysaturated fatty acids. This process leads to lipid peroxidation products, which are responsible for cellular damage as a result of oxidative stress (Halliwell & Gutteridge, Citation1999). Hence, inhibition of XO activity may be important in inhibiting oxidative stress mechanisms that are related to degenerative diseases (Cos et al., Citation1998; Lavelli et al., Citation2000). The purpose of this research is to determine in vitro. xanthine oxidase inhibitory activity of methanol extracts of OS leaves cultivated at different locations in the country as well as to estimate the SEN and TMF contents by high-performance thin-layer chromatography (HPTLC).
Materials and Methods
Chemicals
Sinensetin (SEN) and 3′-hydroxy-5,6,7,4′-tetramethoxyflavone (TMF) were purchased from Indofine Chemical Co. (Hillsborough, NJ, USA). The purity of the commercial compounds (SEN and TMF) were verified by HPLC analysis using the following experimental conditions: LiChrosorb RP-18 column (250 × 4.6 i.d. mm, 10-µm particle size; Merck, Darmstadt, Germany); methanol–water–tetrahydrofuran (45:50:5; v/v) as mobile phase; flow rate of 1 ml/min; injection volume of 20 µl and room temperature. Aluminum chloride, potassium acetate, sodium carbonate, and quercetin, were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals were of analytical grade or HPLC grade.
Plant material
Sample preparation
Plant leaves were ground to a homogeneous powder in a Wiley mill (No. 20 mesh) after drying in an oven (35°C). The dried powdered leaf samples from different locations (1 g each) were extracted with methanol (100 ml) at 40°C for 4 h with continuous stirring. The extracts were filtered through filter paper (Whatman no. 1) with a Buchner filter and cooled to room temperature. The extracts were kept in refrigerator at −20°C until further use.
HPTLC analysis
Chromatography was performed on preactivated (100°C) silica gel 60F254 HPTLC plates (10 × 10 cm; 0.25-mm layer thickness; Merck, Darmstadt, Germany). CAMAG densitometry (Camag Model-3 TLC scanner equipped with Camag CATS 4 software), with a reflectance spectrometer of monitoring range 190–700 nm, was employed for the analysis. The slit was set to 8 × 0.4 mm, and data acquisition and processing were performed using the software winCATS. Samples (20 µl) were applied to the layers at 8-mm-wide bands, positioned 10 mm from the bottom of the plate, using a CAMAG (Mutten, Swizterland) Linomat IV automated TLC applicator with nitrogen flow providing delivery from the string at a speed of 10 µl/s, which was maintained for all analyses. TLC plate development was performed using a CAMAG twin-trough glass tank, which had been presaturated with mobile phase for 2 h. Solvent was allowed to run up the plate to a height of 8 cm. TLC analyses were made under room temperature. A mixture of chloroform:ethyl acetate (6:4) was used as mobile phase. After development, the layers were dried and the components were visualized by UV irradiation at 365 nm. The quantitative determination was performed by winCATS software program using the external calibration method. A stock solution of the standards was prepared in methanol at 1 mg/ml. Standard solutions were prepared by dilution of the stock solution with methanol to give solutions containing the SEN and TMF in the concentration range of 10–1000 µg/ml, respectively. The extracts from different locations (without dilution) were subjected to HPTLC analysis using the method described above.
Determination of total flavonoids and total methanol-soluble contents
Total flavonoid contents of OS were determined by using the aluminum chloride colorimetric method as described by Woisky and Salatino (Citation1998) with some modifications. Methanol extracts (0.5 ml), 10% aluminum chloride (0.1 ml), 1 M potassium acetate (0.1 ml), and distilled water (4.3 ml) were mixed. After incubation at room temperature for 30 min, the absorbance of the reaction was measured at 415 nm. Quercetin was used to develop the calibration curve. The total methanol-soluble content of an aliquot (20 ml) of the extract was determined in triplicate (Joubert, Citation1988).
In vitro. xanthine oxidase inhibition assay
Xanthine oxidase activity with xanthine as substrate was evaluated by spectrophotometric measurement of the formation of uric acid from xanthine (Robak & Grygewski, Citation1988). The primary function of xanthine oxidase is to oxidize xanthine or hypoxanthine to uric acid. Accumulation of uric acid can be monitored spectrophotometrically at 293 nm. The assay mixture consisted of xanthine oxidase (0.5 unit/ml) solution in cold 0.1 M phosphate buffer (pH = 7.5), 0.15 mM xanthine solution in minimal volume of NaOH (pH adjust to 7.5), and OS extract (100 µl in DMSO) in a final volume of 2.8 ml. The mixture was incubated at 37°C for 30 min. Then 1 M HCl (0.2 ml) was added to stop the reaction and the absorbance was read at 293 nm against a blank sample that did not contain the enzyme. A control experiment was performed in the absence of the OS extract. Allopurinol (50 µM) was used as reference inhibitor. Xanthine oxidase activity was expressed as percent inhibition of the xanthine, calculated from the following formula:
Superoxide radical scavenging activity (enzymatic assay)
Superoxide radicals were generated by the X/XO system based on the method of Robak and Grygewski (Citation1988) with some modifications. The reaction mixture contained the same proportion of components as in the xanthine oxidase inhibition assay plus 0.1 mM NBT in a final volume of 3.5 ml. The mixture was incubated for 10 min at 37°C and the coloration of NBT was measured at 560 nm against blank samples that did not contain the enzyme. A control experiment was performed in the absence of OS extract. Quercetin (50 µM) was used as reference compound. Superoxide radical scavenging activity was calculated from the following formula:
IC50 studies
The extract with elevated activity was selected for IC50 (50% inhibitory concentration), studies using the in vitro. methods described above for superoxide scavenging, and xanthine oxidase inhibitory activities. The following concentrations of freeze-dried extracts of OS were used: 2.5, 5, 25, 50, 75, and 100 µg/ml.
Statistical analysis
Experimental results were mean of three parallel measurements and analyzed by SPSS 10 (SPSS Inc., Chicago, IL, USA). Differences between means were determined using Tukey multiple comparisons. p values <0.05 were regarded as significant.
Results and Discussion
HPTLC analysis
The structures of the markers (SEN and TMF) are shown in . The purity of the commercial markers determined by HPLC method was greater than 96%. A mixture of chloroform:ethyl acetate (6:4) used as mobile phase gave a good resolution of SEN and TMF together with symmetrical and reproducible peaks at Rf 0.45 and 0.28, respectively. A and B show the HPTLC profiles of the markers (SEN and TMF) and OS samples. Linear regression analysis for each of the markers was performed by external standard method. Excellent linearity was observed between peak areas and concentrations (r2 > 0.9991) over the range 10–1000 µg/ml for each of the markers. The precision of the HPTLC instrumentation was checked by repeated scanning of the same spots of different concentrations of the markers (10, 100, and 1000 µg/ml) six-times each on a same day (intra-day precision) and on 5 consecutive days (inter-day precision), and the relative standard deviations values were calculated. The results showed very good precision ranging from 10 to 1000 µg/ml. The relative standard deviation (RSD) of the inter- and intra-day precisions of the method was less than 1%. The recovery rates determined at three different concentrations of the markers (10,100, and 1000 µg/ml) to the extracts and analyzed quantitatively in triplicate were in the range 96–99% by the HPTLC assay. The UV-Vis absorption spectra recorded on the CAMAG TLC scanner at the start, middle, and end position of the SEN and TMF bands were superimposable, indicating the purity of the SEN and TMF peak.
Figure 2 HPTLC profiles of methanol extracts of Orthosiphon stamineus.. (A) Two-dimensional chromatogram and; (B) Three-dimensional chromatogram. Eluent: chloroform:ethyl acetate (6:4, v/v); detection: 365 nm.
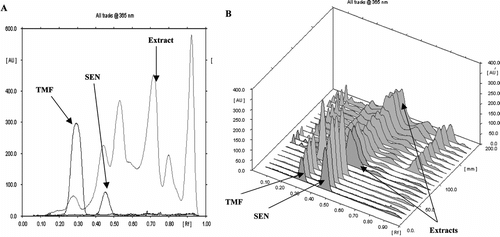
The HPTLC procedure was used as fast screening method for the samples collected from different locations. Qualitatively, similar HPTLC fingerprints were obtained for all the extracts from different locations giving reliable indication of the same identity (B). The two lipophillic flavones in OS leaves, SEN and TMF, used as markers were separated by the HPTLC method and detected in all the samples at Rf 0.45 and 0.30, respectively (B). Using the HPTLC and the UV-Vis spectra techniques, the concentrations of SEN and TMF in the extracts were found to vary from 0.09% to 0.36% and 0.02% to 0.10% of dry weight respectively (). The variation may be due to environmental conditions, which can modify the constituents of the plant.
Table 1 Methanol-soluble solids, total flavonoid content, and content of polyphenol markers in Orthosiphon stamineus. leaf samples collected from different locations of Malaysia between 20 September 2002 and 25 July 2003.
Total flavonoids
The yields of extracts obtained from OS using methanol varied from 7.10% to 18.20%. The result of total flavonoid contents of the methanol extracts of OS from different locations is given in . The total flavonoid contents varied from 3.76 to 5.10 mg quercetin equivalent/g dry weight.
Xanthine oxidase inhibitory and superoxide radical scavenging activities
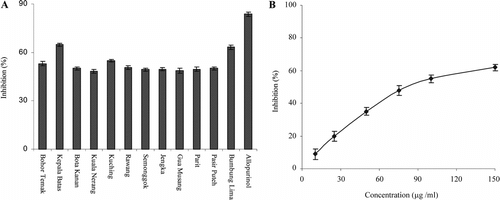
A shows xanthine oxidase inhibitory activities of the freeze-dried extract of OS leaf sample. All the extracts showed XO inhibitory activity, which varied from 48.3% to 64.8%. The extract from location II showed dose-dependent effect on xanthine oxidase inhibitory activity (B) and the IC50 value was 78.4 µg/ml. The variation may be ascribed to the number and position of glycosyl groups on flavonoids present in the extracts, rendering them too bulky and hydrophilic, resulting in decrease in inhibition. The xanthine oxidase inhibitory activity of the extract was lower than the reference inhibitor, allopurinol (100 µM).
Figure 3 (A) Xanthine oxidase inhibitory activity of methanol extracts of Orthosiphon stamineus. leaf; (B) Dose-dependent activity of the extract from site II, Kepala Batas. Values are the means±SD (n = 3).
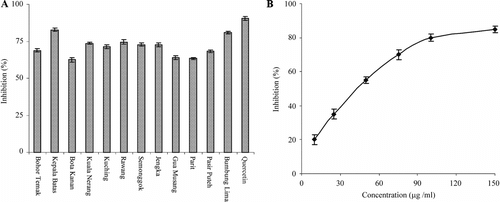
Xanthine oxidase is one of the main oxidative enzymes generating superoxide anion in tissues. Generation of superoxide anions by the xanthine–xanthine oxidase system was measured by the reduction of NBT (yellow) to diformazan (purplish-blue). A shows superoxide radical scavenging activity of the freeze-dried extract of OS leaf sample. The superoxide scavenging activity varied from 63.3% to 82.6%. The activity of the extract was comparable to the reference compound, quercetin (100 µM). The extract from location II showed dose-dependent effect on superoxide anion scavenging activity (B) and the IC50 value was 46.9 µg/ml.
Figure 4 (A) Superoxide anion (NBT/X/XO system) scavenging activity of methanol extracts of Orthosiphon stamineus. leaf; (B) Dose-dependent activity of the extract from site II, Kepala Batas. Values are the means±SD (n = 3).
The activities of the extract may be partly ascribed to the apigenin and luteolin derivatives present in OS leaf, that have been reported to be strong inhibitors of xanthine oxidase (Noro et al., Citation1983). Superoxide and hydrogen peroxide serve as precursors of singlet oxygen and hydroxyl radicals; super oxide indirectly initiates peroxidation of lipids (Kellog & Fridovich, Citation1975). It has also been reported that superoxide can directly initiated lipid peroxidation (Goldstein & Weissman, Citation1977). Xanthine oxidase is considered to be an important biological source of superoxide radicals, and inhibition of xanthine oxidase is an effective therapeutic approach for hyperuricemia causing gout and kidney stones (Lavelli et al., Citation2000). Phenolics inhibit xanthine oxidase and have superoxide scavenging activities, which may contribute to a remedy for human gout and ischemia by decreasing both uric acid and superoxide concentrations in human tissues (Sichel et al., Citation1991). Traditionaly, the leaf extract of the plant is used in the treatment of kidney diseases and gout; this may be linked to the ability of the extracts to inhibit xanthine oxidase and scavenge superoxide anions.
In conclusion, crude methanol extract of OS has been shown to exhibit xanthine oxidase inhibitory action and superoxide radical scavenging activity using in vitro. methods. Therefore, the leaves of the plant may contribute to prevention of gout and kidney stones by its antioxidant effect. The HPTLC method proved useful for the identification of the expected markers in the complex phenolic compounds in the extracts. The HPTLC fingerprint could be used for quality and stability testing of herbal medicinal products containing OS. An in vivo. study to confirm the in vitro. results is in progress in the laboratory.
Acknowledgment
The study was supported by Intensifying Research Priority Areas (IRPA) Grant from Ministry of Science and Technology and Environment, Malaysia.
References
- Cos P, Ying L, Calomme M, Cimanga K, Von Poel B, Pieters L, Vlietinch AJ, Berghe DV (1998): Structure activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod 61: 71–76, [PUBMED], [INFOTRIEVE]
- Goldstein IM, Weissman G (1977): Effects of the generation of superoxide on the permeability of liposomes. Biochem Biophys Res Commun 75: 604–609, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Halliwell B, Gutteridge JMC (1999): Free Radicals in Biology and Medicine, 3rd ed. New York, Oxford University Press, p. 936.
- Hegnauer R (1966): Chemotaxonomic der Planzen, Vol. IV. Stuggart, Birkhäuser Verlag, pp. 314–316.
- Joubert E (1988): Effect of batch extraction conditions on yield of soluble solids from Rooibos tea. Int J Food Sci Tech 23: 43–47.
- Kellog EW, Fridovich I (1975): Superoxide, hydrogen peroxide and singlet oxygen in lipid peroxidation by xanthine oxidase system. J Biol Chem 250: 8812–8817.
- Lavelli V, Peri C, Rizzolo A (2000): Antioxidant activity of tomato products as studied by model reactions using xanthine oxidase, myeloperoxidase, and copper-induced lipid peroxidation. J Agric Food Chem 48: 5630–5639, [CROSSREF]
- Lyckander IM, Malterud KE (1996): Lipophilic flavonoids from Orthosiphon spicatus. prevent oxidative inactivation of 15-lipoxygenase. Prostaglandins Leuko Essent Fatty Acids 54: 239–246, [CROSSREF]
- Noro T, Oda Y, Toxohio M, Uneno A, Fukushima S (1983): Inhibition of xanthine oxidase from the flowers and buds of Dalphne genkwa.. Chem Pharm Bull 31: 3984–3987, [PUBMED], [INFOTRIEVE]
- Robak J, Grygewski RJ (1988): Flavonoids are scavengers of super oxide anions. Biochem Pharmacol 37: 837–841, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Schut GA, Zwaving JH (1993): Pharmacological investigation of some lipophilic flavonoids from Orthosiphon aristatus.. Fitoterapia 64: 99–102.
- Sichel G, Corsaro C, Scalia M, Di Bilio AJ, Bonomo RP (1991): In vitro. scavenger activity of some flavonoids and melanins against O2−. Free Radic Biol Med 11: 1–8, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Tezuka Y, Stampoulis P, Banskota AH, Awale S, Tran KQ, Saiki I, Kadota S (2000): Constituents of the Vietnamese medicinal plant Orthosiphon stamineus.. Chem Pharm Bull 48: 1711–1719, [PUBMED], [INFOTRIEVE]
- Wagner H (1982): Parmazeutische Biologie: Drogen und ihre Inhaltsstoffe, 2nd ed. Stuggart, Gustav Fischer Verlag, pp. 49–50.
- Woisky RG, Salatino A (1998): Analysis of propolis: Some parameters and procedures for chemical quality control. J Agric Res 37: 99–105.