ABSTRACT
Decoctions of the root of the plant Neorautanenia mitis. (A. Rich) Verde (Papilonaceae) are commonly employed ethnomedicinally in the treatment of neuropsychiatric disorders and some other conditions. The central inhibitory effects of the methanol extract of N. mitis. (5–20 mg/kg) were therefore investigated in mice and rats to provide scientific evidence for some of these claims. Activities of the extract were studied on pentobarbital hypnosis in rats, and spontaneous motor activity (SMA), exploratory activity, rota-rod performance (motor coordination), and amphetamine-induced as well as apomorphine-induced hyperactivity, all in mice. The extract at doses of 5, 10, and 20 mg/kg (i.p.) produced significant (p < 0.05) prolongation of pentobarbital hypnosis and significantly (p < 0.05) reduced both SMA and exploratory behavior at the same doses. The extract attenuated both amphetamine- and apomorphine-induced hyperactivity in mice at the higher doses (10 and 20 mg/kg) but had no effect on motor coordination. The results suggest that the extract possesses biologically active principles with central effects that are sedative in nature and may partly be mediated through activity on dopamine (D2)-receptors.
Introduction
Medicinal plants have been used from ancient times to modify and alter mood and feelings. Plants with psychotropic activity are botanically diverse in nature and elicit specific kinds of effects on the central nervous system (CNS). Quite a number of medicinal plants are documented to possess useful activity in mental illnesses. For instance, Piper guineense. (Schumach. and Thonn) seeds (Piperaceae), Cymbopogun citratus. (C. DC.) Stapf. graminae, and Lonchocarpus cyanescens. (Schumach. and Thonn) Benth. (Addac-Mensah et al., Citation1977). Our colleagues (Wambebe et al., Citation1997) have earlier confirmed that cycleanine, an alkaloid extract from the root of Synclisia scabridav. Miers, has remarkable sedative effects that correlate with the traditional application of the plant. Furthermore, the crude extracts of Cistancle deserticola. (MA.) and Diospyros mespiliformis. Hochst. have shown sedative properties (Lu, Citation1998; Adzu et al., Citation2002), corroborating their use as antipsychotics in traditional medicine. Aqueous extracts of Ficus platyphylla. Del Holl. used traditionally to calm agitated people and psychotic patients have also been studied for behavioral effects (Gamaniel et al., Citation2000). The results reveal the psychotropic values of plant extracts.
Neorautanenia mitis. (A Rich) Verde (Papilonaceae) is a perennial herb that grows widely in the middle-belt region of Nigeria and other parts of the African continent, particularly West and Central Africa. In traditional medicine, an alcohol decoction of the tuberous root of this plant is used in the treatment of scabies and has been found to be 100% effective against mites in humans (Heyndrickx et al., Citation1992). Other ethnomedicinal uses of the root include treatment of dysmenorrhea, employed as a cold-water decoction, neuropsychiatric disorders, and as an anticonvulsant. Studies have also shown that the methanol root extract of this plant possesses antinociceptive activity that may be both peripherally and centrally mediated (Vongtau et al., Citation2004). There is currently no scientific documentation of the claimed behavioral or central inhibitory effects of this plant. The objective of the current study was therefore to evaluate pharmacologically the alleged central inhibitory effects of the methanol extract of N. mitis. in mice and rats.
Materials and Methods
Plant material and preparation of extract and drugs
Fresh root tubers of N. mitis. were collected from Suleja, Niger State, in Nigeria between the months of November and January. The plant was botanically identified and authenticated by B. Schrire of the Kews Garden, London. A voucher specimen was made and deposited at the garden's Herbarium for future reference (tag no. H/162/98; collection no. 3942). The root tubers were cleaned, cut into pieces, air-dried for 7 days, and crushed into coarse powder. The powdered root (1 kg) was cold macerated with 2.5 l of methanol for 36 h with constant shaking using a GFL shaker (No. 3017 Gesell Schaftfur Labortechnik mbH, Burgwedel, Germany). The resultant mixture was filtered using Whatman filter paper no. 1 (cat. no. 1001 125) and the filtrate concentrated to dryness in vacuo. at 40○C using a rotary evaporator to give a yield of 6.32% w/w of the extract.
Experimental animals
Male and female Wistar rats (180–250 g) and male and female albino mice (18–25 g) were used in the study. They were obtained from the Animal Facility Centre of the National Institute for Pharmaceutical Research and Development, Abuja, Nigeria. They were fed with standard mouse cubes (Pfizer Feeds) and given water ad libitum.. All experiments performed on laboratory animals in this study followed the “Principles of Laboratory Animal Care” (NIH Publication No. 85, rev. 1985).
Chemicals and drugs
Drugs and chemicals used during the course of the experiments include apomorphine, amphetamine, pentobarbitone, diazepam, and methanol (all from Sigma Chemical Co., St. Louis, MO, USA).
Phytochemical screening
The extract was screened for the presence of various secondary metabolites and constituents using conventional protocols for detecting the presence of alkaloids, tannins, and so forth (Trease & Evans, Citation1983).
Determination of the median lethal dose
The median lethal dose (LD50) was determined in mice and rats both i.p. and p.o. employing the method described by Lorke (Citation1983). Male and female mice and rats were fasted overnight after which the evaluation was carried out in two stages. In the first stage, 3 groups of 6 mice each were treated with the extract at 10, 100, and 1000 mg/kg i.p. in order to discover the range in which the LD50 falls, and this was similarly conducted in 3 groups of 6 rats each. In the second stage, another 3 groups of mice and 3 groups of rats were further treated i.p. with the extract at 25, 50, and 100 mg/kg. In a similar study, the extract was administered orally (p.o.) to the mice and rats. Animals were observed for 24 h after treatment for clinical signs and symptoms of toxicity. The number of deaths in each group was recorded, and the final LD50 values were calculated as the geometric mean of the highest nonlethal dose (with no deaths) and the lowest lethal dose (where deaths occurred).
Spontaneous motor activity (SMA) in mice
Four groups of 6 mice each were used for the study. Group I, which served as a positive control, received distilled water at a dose of 10 ml/kg, and mice in groups II, III, and IV received the extract at doses of 5, 10, and 20 mg/kg i.p., respectively. Thirty minutes after administration of these drugs, the animals were transferred individually into Letica Activity Cages (LE 886) connected to a multicount (LE 3806) and, after a 1-min latency period, activity counts were recorded for 6 min (Wambebe et al., Citation1997).
Pentobarbitone-induced hypnosis in rats
This test was performed in 5 groups of 6 rats each. Three groups received the extract at the doses of 5, 10, and 20 mg/kg i.p. while distilled water (10 ml/kg) was administered (i.p.) to the fourth group, which served as control. The last group received diazepam, i.p. (1 mg/kg). Thirty minutes after this treatment, pentobarbitone sodium (25 mg/kg i.p.) was administered to each rat. Each rat was observed for the onset and duration of sleep, with the criterion for sleep being loss of righting reflex (Wambebe, Citation1985; Roland et al., Citation1991), and the interval between loss and recovery of righting reflex was used as the index of duration of hypnotic effect.
Hole-board test for exploratory behavior in mice
The method used in this experiment was similar to that of Perez et al. (Citation1998). A Letica Board (LE 3333) of 60 cm × 30 cm with 16 evenly spaced holes was used. Thirty mice were divided into 5 groups of 6 mice each. Group one served as control and received distilled water at a dose of 10 ml/kg i.p. Groups II, III, and IV received the methanol extract of N. mitis. at doses of 5, 10, and 20 mg/kg i.p., respectively, and animals in group V were pretreated with diazepam (1 mg/kg i.p.). The animals were placed singly in the arena of the Letica Board 30 min after pretreatment. The instrument automatically counted the number of times the mice dipped their heads into the holes during a 5-min period.
Amphetamine and apomorphine-induced hyperactivity in mice
Adult mice (male and female) were divided into 4 groups of 6 mice each. Group I, which served as control, received normal saline at a dose of 10 ml/kg, and animals in groups II, III, and IV received the extract at doses of 5, 10, and 20 mg/kg i.p., respectively. Thirty minutes after pretreatment, each of the 24 mice were administered amphetamine (2 mg/kg i.p.). They were then transferred individually into Letica Activity Cages (LE 886) connected to a multicounter (LE 3806), and after a 1-min latency period, activity counts were recorded for 6 min (Wambebe et al., Citation1997). In a similar study, the mice were administered apomorphine (2 mg/kg) 30 min after pretreatment with distilled water and extract (5–20 mg/kg), and the activity count similarly recorded.
Test for motor coordination (rota-rod test)
A rota-rod treadmill device (Ugo Basile no. 7650, Italy) was used in conducting this experiment. Mice were trained to remain on slowly moving (16 revolutions/minute) rods of 5-cm diameter for 180 s by walking. Mice that could remain on the rod for 180 s or longer were selected and divided into 4 groups of 6 mice each. Group I served as control and received distilled water at a dose of 10 ml/kg. Groups II, III, and IV received the crude extract of N mitis. at doses of 5, 10, and 20 mg/kg i.p., respectively. Thirty minutes after receiving the injection, animals were singly placed on the rod at intervals of 30 min, up to 120 min. If an animal failed more than once to remain on the rod for 3 min, the test was considered positive, meaning there was a lack of motor coordination (Adzu et al., Citation2002).
Statistical analysis
Results were expressed as mean ± SEM. Data were statistically analyzed using two-way ANOVA followed by Dunnet's post hoc. test for multiple comparisons employing Microsoft Excel software. Differences between means were considered significant when p < 0.05.
Results
Phytochemical screening
Positive reactions were obtained from the extract for the following secondary metabolites: alkaloids, flavonoids, saponins, saponin glycosides, and tannins. Digitalis glycosides, glycosides, phenol, and volatile oils were absent.
Acute toxicity studies in mice: LD50
The LD50 of the methanol extract of N. mitis. was calculated to be 70.71 ± 14.52 mg/kg i.p. and 565.69 ± 116.17 mg/kg p.o. in mice; and values for rats were 74.83 ± 13.87 mg/kg (i.p.) and 692.82 ± 43.42 mg/kg (p.o.). Behavioral changes observed in the animals in the course of the studies were decreased mobility, respiratory distress (gasping) with eventual immobility, but no convulsions or loss of righting reflex prior to death.
Spontaneous motor activity
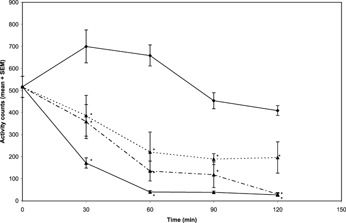
The methanol extract of N. mitis. (5, 10, and 20 mg/kg i.p.) caused a dose- and time-dependent decrease in spontaneous motor activity in mice (). Values were found to be significant [F(3, 23) = 5.73;p < 0.05] over the entire time period of evaluation (i.e., 30, 60, 90, and 120 min) at all the doses tested.
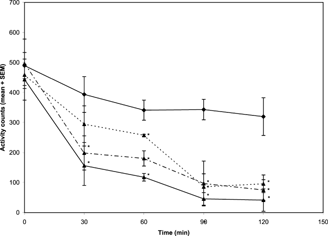
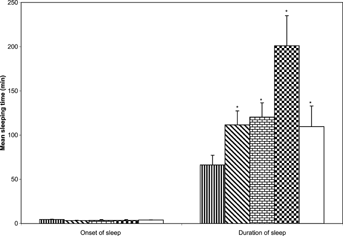
Pentobarbitone-induced hypnosis
The methanol extract of N. mitis. produced significant [F(3, 23) = 12.26;p < 0.05] dose-dependent prolongation of the duration of pentobarbitone-induced hypnosis with no significant (p > 0.05) effects on the onset of sleep ().
Figure 2 Effects of the methanol extract of N. mitis. root (p.o.) 5 mg/kg (![]()
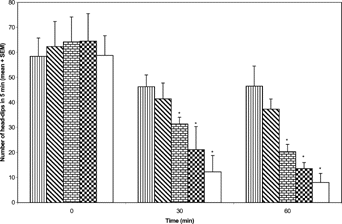
Hole-board test for exploratory behavior
The methanol extract of N. mitis. (5, 10, and 20 mg/kg i.p.) produced a dose-dependent decrease in exploratory activity in mice that was also time-related (). Significant [F(3, 23)=4.31;p < 0.05] differences were observed with both extract (10 and 20 mg/kg) and diazepam (1 mg/kg) compared to control over the period of the study.
Amphetamine- and apomorphine-induced hyperactivity
The extract attenuated amphetamine-induced hyperactivity at 10 and 20 mg/kg significantly [F(3, 23) = 4.05;p < 0.05] and also reduced apomorphine-induced hyperactivity significantly [F(3, 23) = 3.97;p < 0.05] at 20 mg/kg in mice ( and , respectively).
Figure 4 Effects of the methanol extract of N. mitis. root (p.o.) 5 mg/kg (![]()
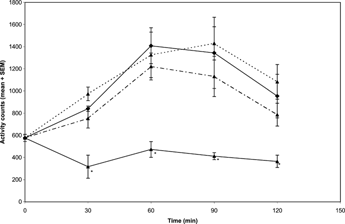
Figure 5 Effects of the methanol extract of N. mitis. root (p.o.) 5 mg/kg (![]()
Test for motor coordination (rota-rod test)
The extract at doses of 5, 10, and 20 mg/kg had no effect on motor coordination as observed in the rota-rod test. Animals in all groups were able to maintain their balance for more than 180 s, which was taken as the cutoff time for each recording at 30, 60, 90, and 120 min for the duration of the experiment.
Discussion
The methanol extract of N. mitis. significantly reduced spontaneous motor activity in mice in this study. Spontaneous motor activity is an acceptable model in evaluating gross behavioral effects of drugs in the laboratory animals (File & Fernandes, Citation1994). Decrease in spontaneous motor activity indicates reduction in CNS excitability and depression of the CNS (Ozturk et al., Citation1996). Therefore, the extract is likely sedative in nature. The extract also prolonged pentobarbitone-induced hypnosis in rats. This prolongation of pentobarbitone-induced hypnosis observed in this study strongly suggests central depressant activity of the extract (Perez et al., Citation1998) and supports the suggestion that the extract may be sedative.
The increase in pentobarbitone-induced sleeping time could be due to inhibition of pentobarbitone metabolism (Kaul & Kulkarni, Citation1978) or an action related to the central mechanism involved in regulation of sleep. For instance, endogenous neurotransmitters in the brain, particularly dopamine and GABA, have been implicated in sleep mechanisms (Osuide & Wambebe, Citation1980; Gottesmann, Citation2002). It is therefore possible that the extract of N. mitis. produced these effects via augmentation of the central inhibitory effect of GABA or through dopaminergic pathways. The effects could also result from interference with the excitatory mechanism of the reticular activating system.
The extract of N. mitis. also caused a significant decrease in the exploratory behavior pattern as shown by the results of the head dip in the hole-board test. Decrease in head-dipping further reveals sedative effects (File & Pellow, Citation1985) as opposed to anxiolytic activity, which has been shown to cause an increase in this parameter (Takeda et al., Citation1998).
Amphetamine induces hyperactivity by releasing biogenic amines, particularly dopamine, from the dopaminergic nerve terminals in the striatal pathways of the mesolimbic system (Hoffman & Lefkowitz, Citation1996), whereas apomorphine acts directly on the postsynaptic dopamine receptors similar to dopamine. The effects of the extract on amphetamine- and apomorphine-induced hyperactivity suggest possible interference with central dopaminergic transmission. It has actually been established that the dopaminergic system, particularly in the striatum and nucleus accumbens, has high concentrations of dopamine (D2)-receptors that in the CNS are involved in locomotor activity (Andien & Grabowska-Andien, Citation1989). Therefore, the central effects that are sedative in nature may partly be mediated through activity on D2-receptors.
Effects of the crude methanol extract on the treadmill performance of mice were investigated in the rota-rod test in order to ascertain its effect on physical performance and possible neuromuscular inhibition. The extract did not affect the performance test, indicating that the extract might not have peripheral neuromuscular blocking effects. Rather, results suggest involvement of central mechanism in the actions of the extract (Perez et al., Citation1998).
In conclusion, this study has shown that the methanol extract of N. mitis. root possesses central effects that are sedative in nature. These effects may likely be partly mediated via D2-receptors as is suggested by attenuation of hyperactivity induce by amphetamine and apomorphine. The study has therefore provided scientific evidence to show central depressant effects of N. mitis., thereby scientifically explaining and corroborating the traditional use of this plant, as well as confirming the presence of potentially useful pharmacologically active principles. Studies are currently underway to isolate and characterize the active constituent(s) of this plant.
Acknowledgment
This work was made possible by funding from the National Institute for Pharmaceutical Research and Development (NIPRD), Abuja, Nigeria. The authors gratefully acknowledge the technical assistance of Hauwa Abdullahi, Sunday Dzarma, Zacharia Tags, and Adamu Mohammed.
References
- Addac-Mensah I, Torto FG, Dimonyeka CL, Baxter I, Sanders KM (1977): Novel amide alkaloids from the roots of Piper guiniense.. Phytochemistry 16: 757.
- Adzu B, Amos S, Dzarma S, Muazzam I, Gamaniel KS (2002): Pharmacological evidence favouring the folkloric use of Diospyros mespiliformis. Hochst in the relief of pain and fever. J Ethnopharmacol 82: 191–195. [PUBMED], [INFOTRIEVE], [CROSSREF], [CROSSREF], [CSA]
- Andien NE, Grabowska-Andien M (1989): Stimulation of post synaptic D2 dopamine receptors by B-HT 958 is revealed by co-treatment with the D1 receptor agonist SRF 38393. J Pharmacol 41: 490.
- File SE, Fernandes C (1994): Dizocilpine prevents the development of tolerance to the sedative effects of diazepam in rat. Pharmacol Biochem Behav 47: 823–826. [PUBMED], [INFOTRIEVE], [CROSSREF], [CROSSREF], [CSA]
- File SE, Pellow S (1985): The effect of triazolobenzodiazepine in two animal tests of anxiety and on the hole board. Br J Pharmacol 86: 729–735. [PUBMED], [CSA]
- Gamaniel K, Amos S, Chindo B, Wambebe C, Vongtau H, Olusola A, Abdulrahman EM, Odutola AA, Akah PA, Adamu SS (2000): Behavioural effects of the methanol extract of Ficus platyphylla. bark in mice and rats. Nig J Neurosci 3: 17–23. [CSA]
- Gottesmann C (2002): GABA mechanisms and sleep. Neuroscience 111: 231–239. [PUBMED], [INFOTRIEVE], [CROSSREF], [CROSSREF]
- Heyndrickx G, Briven P, Von Puyvelde L (1992): Study of Rwandese medicinal plants used in the treatment of scabies. J Ethnopharmacol 35: 259–262. [PUBMED], [INFOTRIEVE], [CROSSREF], [CROSSREF]
- Hoffman BB, Lefkowitz RJ (1996): Catecholamines, sympathomimetic drugs and adrenergic receptor antagonists. In: Hardman JG, Limbird LE, Molinoff PM, Ruddon RW, Gillman AG, eds., Goodman and Gilman's Pharmacological Basis of Therapeutics, 9th ed. New York, McGraw-Hill, pp. 199–248.
- Kaul PN, Kulkarni SK (1978): New drug metabolism inhibitor of marine origin. J Pharm Sci 67: 1293–1295. [PUBMED], [INFOTRIEVE]
- Lorke D (1983): A new approach to acute toxicity testing. Arch Toxicol 54: 275–287. [PUBMED], [INFOTRIEVE], [CROSSREF], [CROSSREF]
- Lu MC (1998): Studies on the sedative effect of Cistarche desuficola.. J Ethnopharmacol 59: 161–165. [PUBMED], [INFOTRIEVE], [CROSSREF], [CROSSREF], [CSA]
- Osuide G, Wambebe C (1980): Antagonism of pentobarbitone sleep by dopamine, levodopa and apomorphine in chicks. Clin Exp Pharmacol Physiol 7: 237–248. [PUBMED], [INFOTRIEVE]
- Ozturk Y, Aydine S, Beis R, Baser KHC, Bergeroglu H (1996): Effects of Hypreicum perforatum. L. and Hypericum calicynum. L. extracts on the central nervous system in mice. Phytomed 3: 139–146.
- Perez GRM, Perez IJA, Garcia D, Sossa MH (1998): Neuropharmacological activity of Solanum nigrum. fruit. J Ethnopharmacol 62: 43–48. [PUBMED], [INFOTRIEVE], [CROSSREF], [CROSSREF], [CSA]
- Roland A, Fleurentin A, Lanhers M, Younous C, Missilin R, Morrier F (1991): Behavioural effects of American traditional plant Escholzia californica., sedative and anxiolytic properties. Planta Med 57: 212–216. [CSA]
- Takeda H, Tsuji M, Matsumiya T (1998): Changes in head-dipping behaviour in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur J Pharmacol 350: 21–29. [PUBMED], [INFOTRIEVE], [CROSSREF], [CROSSREF]
- Trease GE, Evans MC (1983): Textbook of Pharmacognosy, London, Balliere, Tindall, pp. 343–383.
- Vongtau HO, Abbah J, Mosugu O, Chindo BA, Ngazal IE, Salawu AO, Kwanashie HO, Gamaniel KS (2004): Antinociceptive profile of the methanol extract of Neorautanenia mitis. root in rats and mice. J Ethnopharmacol 92: 317–324. [PUBMED], [INFOTRIEVE], [CROSSREF], [CROSSREF]
- Wambebe C (1985). Influence of some agents that affect 5-HT metabolism and receptors and nitrazepam-induced sleep in mice. Br J Pharmacol 84: 185–191. [PUBMED], [INFOTRIEVE]
- Wambebe C, Gamaniel K, Akah P, Kapu SD, Samson A, Orisadipe AT, Okogun JI (1997): Central and uterotonic effects of cycleanine. Indian J Pharmacol S366–S372.