Abstract
The in vitro. antifungal activity of aqueous, ethanol, chloroform, petroleum ether, and residue extracts from 10 indigenous Bahraini plants used in folk medicine for the treatment of various diseases is reported. Extract efficacy was evaluated using the agar well diffusion assay against four filamentous fungi and two yeasts monitored by standard antifungal disks. The results showed that all but, in particular, ethanol and chloroform plant extracts reveal variable degrees of bioactivity against at least two of the tested microbes. The highest ethanol extract activity was exhibited by Cressa cretica. (L.) against Penicillium citrinum. Thom (32.2 mm) followed by Candida albicans. (C. P. Robin) Berkhout (25.7 mm). The diffusable metabolites of Heliotropium curassavicum. L. also demonstrated marked inhibitory effects against the same microorganisms. Chloroform extracts of Emex spinosa. Campd. displayed an elevated potency against Alternaria alternata. (Fries) Keissler (27.9 mm) and Saccharomyces cerevisiae. Meyen ex. E. C. Hansen (27.5 mm). Zone of inhibition against other fungi varied from 19.9 to 25.9 mm. However, the highest growth inhibition was encountered with Fagonia indica. Burm F. against P. citrinum. (29.3 mm). With the exception of chloroform extracts from cultivated soils, various extracts of plants randomly collected from saline-affected soils exhibited higher fungal radius inhibition than plants from cultivated soils. The significance of these results in relation to ethnobotanical data is discussed.
Introduction
Bahrain is a small, semiarid island nation in the Arabian Gulf with rich plant resources. About 310 plant species have been reported from various ecological habitats (El-Oqlah & Abbas, Citation1994). Of these, 80 species have been documented as folk remedies for both internal and external use (Abbas & Al-Saleh, Citation2002). The people of Bahrain have a very long-standing tradition in the trade and use of ethnomedicine due to the island's strategic location and several other sociocultural factors. The practice is still strong in the treatment of minor ailments with these plants including ulcers, pneumonia, stomach disorders, rheumatism, diabetes, renal problems, and bronchitis (Abbas et al., Citation1992). Researchers are increasingly turning their attention to folk medicine and antimicrobial compounds from plant species used in herbal medicine in Bahrain (Abbas et al., Citation1992; Al-Saleh et al., Citation1993, Citation1997; Mahasneh et al., Citation1996; Taha & Al-Sayed, Citation2000). Moreover, herbal medicine has improved as an alternative effective solution to health problems and cost of pharmaceutical products. However, little work has been done to match this ethnobotanical information with analytical research to identify active chemical compounds.
Plants used in traditional medicine can offer potential sources of new biological active compounds, many of them as anticancer, anti-HIV, and antifungal agents. Examples of these compounds include flavanoids, saponins, phenolics, glucosinolates, and cyanogenic glycosides (Bennett & Wallsgrove, Citation1994; Grayer & Harborne, Citation1994). Also, these plants can serve as a source of model compounds for synthetic or semisynthetic structure modification (Balandrin et al., Citation1993). Potential natural or synthetic substances with biocidal activity are considered candidates for developing new drugs for the treatment of various chronic as well as infectious diseases.
In hot humid countries like Bahrain, human infection by fungi, especially those on skin, vaginal, and mucosal surfaces, constitute a serious problem. Furthermore, the number of reported cases of immunocompromised and HIV-infected patients with opportunistic and superficial mycoses like cryptococcosis, candidiasis, and aspergillosis has increased in recent years (World Health Organization, Citation1998). The use of several antimycotic drugs available at present is limited by the emergence of new resistant strains, toxicity, poor solubility, and low potency (Navarro-Garcia et al., Citation2003). Therefore, it is of prime importance to search for new, safe, and more effective antifungal agents. In this connection, indigenous medicinal plants continue to be a rich source of therapeutic drugs. The active principles of many drugs are usually found in plants as secondary metabolites. Nevertheless, relatively few studies are focused on developing antifungal compounds (Jones et al., Citation2000; Portillo et al., Citation2001; Quiroga et al., Citation2001; Navarro-Garcia et al., Citation2003) from medicinal plants as compared to antibacterial substances (Al-Saleh et al., Citation1997; Mahasneh et al., Citation1996).
The aim of this screening experiment was the selection of promising plant species for further bioactivity-guided fractionation of active antifungal compounds. We report the results of an in vitro. evaluation against fungi of various extracts from 10 indigenous traditional medicinal plants. No data are available on the use of these plants in folk remedies. Also, the therapeutic efficacy and antimycotic activity associated with these plants has not been evaluated previously. It is hoped that the data presented here will not only provide useful scientific information but also encourage further interest and research on Bahraini medicinal plants.
Materials and Methods
Plant material
Ten plant species were collected in 2002 in their natural habitats from various regions of Bahrain. Dr. D. Al-Esawi (Department of Biological Sciences, Faculty of Science, University of Jordan, Amman, Jordan) identified the plant materials according to the checklist of El-Oqlah and Abbas (Citation1994). Voucher specimens were deposited in the herbarium collection of the Department of Biology of the University of Bahrain. Acquisition code numbers are listed in .
Table 1.. Ethnobotanical data of medicinal plants used in antifungal assay.
Preparation of plant extracts
Plant parts, mostly leaves and stems, were shade-dried at room temperature (25–30°C) and later ground into a fine powder using a household blender and then sieved with a 2-mm-diameter mesh. The pulverized material (50 g) was extracted sequentially with ethanol, petroleum ether, and chloroform as follows.
Pulverized air-dried materials (ca. 50 g) were extracted continually for 48 h in a Soxhlet extractor using 200 ml of 96% ethanol. The insoluble material was filtered off using Whatman filter paper no. 4, and the filtrate was dried (anhydrous MgSO4) and concentrated by complete evaporation of the solvent in a vacuum rotary evaporator at 40°C (water bath temperature). Percent yield of ethanol extract (EE) of each plant material was determined from the resulting residue (0.56–1.46 g). Each of the ethanol extracts, after setting aside a portion for the antifungal assay, were further extracted with petroleum ether (b.p. 60–80°C) three-times (3 × 10 ml). The supernatant solutions were separated by decantation, combined, dried, and the solvent evaporated in the rotary evaporator as above to give the petroleum ether extract. This extract, part of which was used in antifungal test, was further extracted with three portions of chloroform (3 × 10 ml). The mixture was filtered, separated from droplets of water, and dried (anhydrous MgSO4). Complete evaporation of chloroform afforded the residue extract.
In vitro. antifungal test solutions of these extracts and controls were prepared by dissolving the equivalent of 100 mg in 1 ml of 5% dimethylsulfoxide (DMSO, Merck, Germany). The mixture resulted in a homogenous solution that was placed in small vials and stored at 5°C.
For the aqueous extract, 10 g of plant powder was soaked in 50 ml of distilled de-ionized water for 72 h while shaking over a water bath at 40°C. The mixture was left for 3 h at room temperature and then the supernatant was centrifuged at 1000 × g at room temperature. The filtrate was oven dried at 40°C until completely dry. The dried aqueous crude extract was weighed, and the concentration was adjusted to 100 mg/ml with distilled sterilized water.
Fungal cultures and growth conditions
Test fungi used in this study () were chosen primarily on the basis of their importance to plants and human as pathogens. The human pathogens were the yeast Candida albicans. (C. P. Robin) Berkhout (clinical isolate from American Mission Hospital, Bahrain) and Saccharomyces cerevisiae.. Stocks were maintained on Sabouraud's dextrose agar (SDA) (Oxoid, Hampshire, UK) slants at 4°C. The filamentous plant pathogens Alternaria alternata. (Fries) Keissler, Penicillium citrinum. Thom, Aspergillus flavus. Link, Aspergillus niger. van Tieghem, and Fusarium oxysporum. Schlecht were locally isolated from diseased plant parts and identified by standard procedure by the first author. Stock cultures of these fungi were maintained on potato dextrose agar (PDA) (Oxoid) slants at 4°C prior to use in antifungal tests.
Table 2.. In vitro. antifungal activity of crude aqueous and ethanol extracts of 10 Bahrain medicinal plants.
Test for antifungal activity
Antimycotic activity of aqueous, ethanol, petroleum ether, chloroform, and reside extracts of each plant species was evaluated by the agar well diffusion assay as modified by Quiroga et al. (Citation2001).
Overnight cultures of yeasts (C. albicans. and S. cerevisiae.) were prepared by inoculating the organisms in a 250-ml Erlenmeyer flask containing 50 ml Sabouraud's broth medium (SDM). The culture was incubated at 30°C for 18 h. Inocula of filamentous fungi (A. alternata., A. niger., A. flavus., P. citrinum., and F. oxysporum.) were prepared on potato dextrose agar (PDA) in a 250-ml Erlenmeyer flask for 8 days at 30°C in alternate cycles of 12 h light and dark. Spore suspensions were raised by pouring 5 ml of distilled sterilized water into the flask, vortexing for 1 min, and sieving through 8 layers of cheesecloth. A final inoculum density of 105 cell/ml for yeast and 106 spore/ml for the filamentous fungi was calibrated using a hemocytometer.
Aliquots of 50 µl inoculum of either yeasts or filamentous fungi were aseptically mixed with 20 ml melted Sabouraud's dextrose agar; dextrose agar (SDA) or PDA media cooled at 45°C. The media-spore suspension was gently mixed and poured aseptically into 9-cm-diameter plastic Petri dishes. Plates were allowed to stand for 1 h at room temperature and a small well (5 mm) was cut in the middle of each solidified medium using sterilized cork borer. One hundred microliters of each plant extract (100 mg/ml) was slowly loaded in each well using a micropipette. The dishes were then preincubated at 4°C for 2 h to allow uniform diffusion of the extract into the agar. After preincubation, the plates were incubated aerobically at 30°C for 48 h for yeasts and 72 h for filamentous fungi.
Code numbers to maintain objectivity blinded the identity of the plates. Appropriate treatments including wells loaded only with sterilized distilled water or DMSO were considered as negative controls. Additionally, for comparative purposes, disks containing standard antifungal disks like Nystatin and miconazol nitrate (50 µg/ml) served as positive control. Each experiment was carried out in triplicate and repeated at least twice. The results were recorded by measuring the zone of growth inhibition around the agar well that was expressed in mm diameter. The mean of three readings per zone was noted and standard deviation of all replicates determined.
Results
shows the ethnobotanical data, extract yields (%), and collection codes of 10 selected Bahraini medicinal plants. They are represented by nine families and were primarily in the form of herbs or shrubs. Plant parts consisted mostly of leaves and stems except for C. spinosa., where fruits were also included in the assay. This plant resulted in the highest extract yield (6.13%) compared to other plants. Five plants, H. curassavicum., F. pulverulenta., C. cretica., C. spinosa., and T. arabica., were sampled from salt-affected soils, whereas the remaining plants were collected from cultivated locations. Most of these plants have been used in folk remedies in different forms for various afflictions (Abbas et al., Citation1992). Nonetheless, no information of their antifungal application and usage frequency to combat infectious diseases whose etiological origin appears to be microbial or anti-inflammatory has previously been reported. Thus, selection of plants for this study was based on availability of collection.
Bioactivity of the plant extracts was determined with five filamentous fungi and two yeasts using the agar well diffusion method. Results from the methanol and aqueous plant extracts are summarized in . The extent of activity of these extracts was quantitatively assessed by measuring the diameter of zone of inhibition around the well in millimeters. Comparisons of data with standard fungicidal disks were included to monitor the experimental conditions and to facilitate better evaluation of the results with other published reports.
All of the examined plant extracts demonstrated varying degrees of biological activity against at least one of the tested microbes. Ethanol extracts were superior to aqueous crude extracts and showed broader spectrum of activities as shown in . In particular, the ethanol extract of H. curassavicum. was found to be active against all the fungal strains tested. Also, extracts from E. spinosa., F. pulverulenta., C. cretica., P. ovalis., and C. spinosa. proved active against most of the fungi assayed. Overall, the highest inhibitory effect of the ethanol solution was exhibited by C. cretica. against P. citrinum. (32.2 mm) followed by C. albicans. (25.7 mm) and A. alternata. (22.1 mm). Furthermore, the ethanol crude solution of H. curassavicum. showed marked activity toward P. citrinum. (25.5 mm) and the yeasts C. albicans. and S. cerevisiae. with inhibition zones of 21.3 mm and 20.4 mm, respectively. Apart from A. alternata., extracts from P. ovalis. revealed elevated inhibitory effect against all microbes evaluated. Mainly, growth reduction was noted in opposition to C. albicans. (18.9 mm) followed by F. oxysporum. (14.6 mm) and P. citrinum. (14 mm). However, the efficiency of all other ethanol extracts encountered was somewhat intermediate with an average of 12.7-mm inhibition zone. The lowest antifungal activities were reported for F. indica. and C. arvensis..
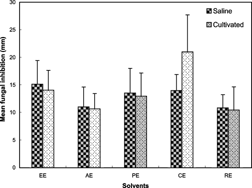
Figure 1. Mean fungal inhibition zone (mm±SD) by each of the two plant habitat types for various solvents used against filamentous fungi and yeasts.
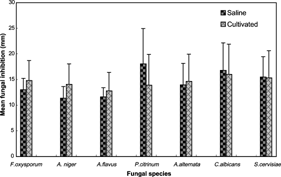
Among filamentous fungi, the highest susceptibility frequency toward all plant extracts was observed with A. flavus. (100%) followed by A. alternata. (80%) and P. citrinum. (70%). Among the yeasts, C. albicans. was more sensitive to plant extracts (80%) than S. cerevisiae. (60%).
Aqueous crude extracts showed, in general, poor bioactivity against the assayed microorganisms (). The inhibitory effect ranged from as low as 8.2 mm for T. arabica. against A. alternata. to as high as 15.6 mm for C. cretica. against P. citrinum..
The results () show that ethanol extracts possess pronounced antimycotic properties and that some plants like C. cretica. and H. curassavicum. retain increased biological attributes. As a result of the above observations, the ethanol extract of some plants were further fractionated. The resulting petroleum ether, chloroform, and residue extracts from H. curassavicum., E. spinosa., F. pulverulenta., F. indica., and C. echinatus. were examined for antifungal activity as show in .
Table 3 In vitro. antifungal activity of petroleum ether, chloroform, and residue extracts of five Bahraini medicinal plants.
All plant extracts revealed some degree of antifungal activity with solvent extracts showing a broad-spectrum activity at 100 mg/ml toward at least two of the fungi assayed. Chloroform extracts of H. curassavicum. and E. spinosa. and the petroleum ether extracts of F. pulverulenta. demonstrated a high efficacy potential against all of the microbes examined. The highest extract potency was displayed by the chloroform extracts, whereas the petroleum ether and residue extracts indicated a similar reduced pattern of inhibition.
Among all the fractionations, the broadest and prominent activity was detected in the chloroform extract of E. spinosa. against A. alternata. (27.9 mm) and S. cerevisiae. (27.5 mm) (). Likewise, the activity against other fungi using the same extract varied from 19.9 to 25.9 mm for P. citrinum. and C. albicans., in that order. The highest zone of inhibition was exhibited against P. citrinum. (29.3 mm) for F. indica., followed by C. albicans. (25.6 mm). The diffused chloroform extracts from the agar wells of H. curassavicum. and C. echinatus. also revealed marked activity with a highest zone of 21 and 20 mm against S. cerevisiae. and A. flavus., respectively. Reduced antifungal activity was encountered with F. pulverulenta..
The crude petroleum ether fractions of three plants, namely, E. spinosa, F. pulverulenta., and C. echinatus., illustrated the highest comparable inhibitory level with an average of 16.2 mm against A. flavus, S. cerevisiae., and P. citrinum.. A pronounced antifungal activity (average 15 mm) was also noted in the petroleum ether extract of H. curassavicum. against A. flavus. and C. albicans. and in the extract of F. indica. against A. alternata. and S. cerevisiae.. Other filamentous fungi and yeasts were also susceptible at different levels toward various plant fractions.
Residue extracts yielded a somewhat similar pattern of reduced microbial growth but showed only narrow-spectrum efficiency. The lowest inhibitory activity was more than 9 mm in radius against A. niger.. The residue extracts of H. curassavicum. attained the highest growth reduction (17.2 mm) against P. citrinum.. This plant and the diffusable extract of C. echinatus. both exhibited analogous inhibition radii of 14.4 mm against A. alternata. and A. niger.. The other residue fractions were active against at least two fungi assayed.
In , the mean inhibitory zone diameter for extracts from each habitat (5 saline and 5 cultivated soils) is illustrated for filamentous fungi and yeasts. For all microbial species, P. citrinum. was the most susceptible microbe toward the overall diffusable metabolites of saline habitat (18.05 mm), followed by the yeasts C. albicans. (16.8 mm) and S. cerevisiae. (15.5 mm). The other yeasts also displayed an increased susceptibility level toward extracts from plants of cultivated soils than did the filamentous fungi.
Figure 2.. Mean fungal inhibition zone (mm±SD) by each of the two plant habitat types against filamentous fungi and yeasts. EE, ethanolic extract; AE, aqueous extract; PE, petroleum ether extract; CE, chloroform extract; RE, residual extracts.
The effect of various solvents from each plant habitat type on mean antifungal activity is shown in . Unlike chloroform extracts from cultivated soils, which revealed elevated antifungal activity, extracts of plants from saline-affected soils showed only slightly increased activity over those from cultivated habitats. In general, the efficacy of solvents can be arranged in the order of magnitudes chloroform > ethanol > petroleum ether > residue > aqueous.
Discussion
This study clearly shows that all but, in particular, chloroform and ethanol extracts of indigenous Bahraini medicinal plants possess substantial yields of ingredients active against the assayed microbes (Tables and ). The yeasts appeared to be quite susceptible to the materials diffused from the ethanol extracts of C. cretica. and H. currasavicum. as revealed by the zone radii of 25.7 and 20.4 mm, respectively (). Likewise, chloroform extracts of both E. spinosa. and F. indica. also proved to be active in restricting the growth of C. albicans. (27.5 mm) while F. indica. was active against S. cerevisiae. (27.5 mm). This observation is of particular interest, as C. albicans. is a ubiquitous pathogen common in pathogenesis of urinary tract infections, endocarditis, vulvovaginalis, and oral thrush (Greenspan & Greenspan, Citation1997). The yeast also causes serious systemic infection, including opportunistic infections in HIV patients (Quiroga et al., Citation2001).
For filamentous fungi, the ethanol diffusable substances of C. cretica. and H. curassavicum. showed prominent biological activity against P. citrinum. at a diameter of restriction of 32.2 and 25.5 mm, respectively (). In addition, the assayed microbes concealed a measurable degree of susceptibility toward the chloroform extracts of E. spinosa. with the greatest inhibition found against A. alternata. (27.9 mm) followed by A. niger. (23.6 mm) (). P. citrinum. was the most sensitive fungus to the diffusable metabolites of F. indica. (29.3 mm). Members of Aspergillus., Altenaria., Penicillium., and Fusarium. are well-known for their production of toxins (e.g., aflatoxins of A. flavus.). These secondary metabolites are potent carcinogens, hepatotoxins, teratogens, and immunosuppressor compounds (Quiroga et al., Citation2001). Species of F. oxysporum. cause important plant diseases like wilts, damping-off, root rot, and seed decay. Species of Fusarium. produce potent mycotoxins in food commodities and are commonly prevalent fungi affecting corn (Marassas, Citation1991). These fungi represent threats not only to plants but also to animals and humans consuming contaminated feed and food.
These findings suggest that the antifungal properties in these plants are most likely due to the presence of broad-spectrum biological compounds or general metabolic inhibitors. Indigenous plants sampled randomly from saline soils demonstrated somewhat higher inhibition levels, except in opposition to P. citrinum. and C. albicans. (). It is probable that plants inhabiting salt-affected soils are more likely weakened by stress conditions caused by salinity as compared to cultivated soil plants, the former being more prone to pathogen attack and thus as a defense mechanism produces antimicrobial secondary metabolites in response. Examples of such synthesized metabolites include tannins, phenolic compounds, and prolines (Rizq, Citation1986; Al-Saleh et al., Citation1993; Bennett & Wallsgrove, Citation1994). Similarly, plants are known to produce antifungal compounds in response to pathogen attack or other abiotic stress factors (Wojtaszek, Citation1997; Osbourne, Citation1998).
With the exception of one study (Mahasneh et al., Citation1996), no reports on the antifungal properties and chemical nature of the inhibitory compounds of the examined plant taxa could be found in the literature. In their study, Mahasneh et al. (Citation1996) reported a mild inhibitory effect (average 11.5 mm) of various extracts of C. spinosa. against F. oxysporum. and C. albicans.. This inhibitory level is comparable to the reported activity in this study of the ethanol extract against the same microorganisms tested ().
Analysis of compounds in many plant families revealed the occurrence of common phytochemical constituents. In this regard, P. ovalis., belonging to Compositae, is well-known for its sesquiterpene lactones as bioinhibitors (Bohlman, Citation1988). Many such lactones are antibacterial agents, especially, against Gram-positive bacteria (Bruneton, Citation1995). E. spinosa. has been reported to contain anthraquinones as well as quercitrin and rutin flavanoids, coumarins, and alkaloids (Rizq, Citation1986). C. cretica. has been reported to contain quercetin glycosides, alkaloids, coumarins, and sterols (Rizq, Citation1986). C. arvensis., known locally as ollaiq., contains saponins, sterols, and alkaloids. F. indica. has been reported to contain triterpenoids, saponins, alkaloids, flavanoids, and tannins (Rizq, Citation1986). Pluchea. spp. also contains quercetin glycosides. H. currassavicum. has been reported to contain pyrrolizidine alkaloids, and F. pulverulenta. has been reported to contain flavanoids, coumarins, and tannins (Rizq, Citation1986). Certain compounds produced by these plants have been reported to have antifungal activity as well. Anthraquinones have been reported to have antifungal efficacy against A. niger. (Ali et al., Citation1999), whereas pyrrolizidine alkaloids have been reported to have antimicrobial activity (Singh, Citation2002).
An understanding of the inhibitory mechanisms of these indigenous Bahraini medicinal plants could provide an avenue toward the development and efficient production of new chemical classes of novel metabolites (Hostettman, Citation1998). This is the first report describing the antifungal activities of different Bahraini medicinal plants. The use of these plants in the treatment of various infectious diseases, whose symptoms might be of fungal origin, highlights the importance of ethnobotanical approach for the future selection of plants in the discovery of new bioactive compounds. Further work, including structure-function relationship and bioactivity-guided fractionation to isolate and purify antifungal compounds in some of these plants, is now in progress.
References
- Abbas JA, Al-Saleh FS (2002): Medicinal Plants of Bahrain, Bahrain, University of Bahrain Press, 290pp.
- Abbas JA, El-Oqlah AA, Mahasneh AM (1992): Herbal plants in the traditional medicine of Bahrain. Econ Bot. 46: 158–163.
- Ali MI, Shalaby NM, ELgamal MH, Mousa, AS (1999): Antifungal effects of different plant extracts and their major components of selected aloe species. Phytother Res 13: 401–407. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Al-Saleh FS, Ali HH, Mirza M (1993): Chemical constituents of some medicinal plants growing in Bahrain. Fitoterapia LXIV: 251–256. [CSA]
- Al-Saleh FS, Gamal El-Din AY, Abbas JA, Saeed NA (1997): Phytochemical and biological studies of medicinal plants in Bahrain: Family Chenopodiaceae—Part 2. Int J Pharmacog 35: 1–5.
- Balandrin MF, Kinghorn AD, Fransworth NR (1993): Plant-derived natural products in drug discovery and development. In: Kinghorn AD, Balandrin MF eds., Human Medical Agents From Plants. Washington, DC: American Chemical Society, pp. 1–12.
- Bennett RN, Wallsgrove RM (1994): Secondary metabolites in plant defense mechanisms. New Phytol 127: 617–633. [CSA]
- Bohlman F (1988): Compositae, a source of unusual natural compounds. GIT Fachz Lab 32: 453–456.
- Bruneton J (1995): Pharmacognosy, Phytochemistry of Medicinal Plants. Paris, France, Lavoisier Publishing, 501 pp.
- El-Oqlah AA, Abbas JA (1994): A checklist of vascular plants of Bahrain. Dirasat 21B (6): 95–118.
- Grayer RJ, Harborne JJ (1994): A survey of antifungal compounds from higher plants, 1982–1993. Phytochemistry 37: 19–42. [CROSSREF], [CSA]
- Greenspan JS, Greenspan D (1997): Oral complication in HIV infections. In: Merle A, Sande MD, PaulA, Volberding MD eds., The Medical Management of AIDS. London, Saunders, pp. 169–180.
- Hostettmann K (1998): Strategy for the biological and chemical evaluation of plant extract. Pure Appl Chem 70: 1–9.
- Jones NP, Arnason JT, Aou-Zaid M, Akpagana K, Sacchez-Vindas P, Smith ML (2000): Antifungal activity of extracts from medicinal plants used by First Nation Peoples of eastern Canada. J Ethnopharmacol 73: 191–198. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Mahasneh AM, Abbas JA, El-Oqlah AA (1996): Antimicrobial activity of extracts of herbal plants used in the traditional medicine of Bahrain. Phytother Res 10: 251–253. [CROSSREF], [CSA]
- Marassas WFO (1991): Toxigenic Fusaria. In: Smith JE, Henderson RE eds., Mycotoxins and Animal Foods. Boca Raton, FL, CRC Press, pp. 119–193.
- Navarro-Garcia VM, Gonzalez A, Fuentes M, Aviles M, Rios MY, Zepeda G, Rogas MG(2003). Antifungal activities of nine traditional Mexican medicinal plants. J. Ethnopharmacol 87: 85–88. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Osbourne AE (1998): Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell 8: 1821–1831. [CROSSREF]
- Portillo A, Villa R, Freixa B, Adzet T, Caigueral S (2001): Antifungal activity of Paraguayan plants used in traditional medicine. J Ethnopharmacol 76: 93–98. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Quiroga EN, Sampietro AR, Vattuone MA (2001): Screening antifungal activities of selected medicinal plants. J Ethnopharmacol 74: 89–96. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Rizq AM (1986): The Phytochemistry of the Flora of Qatar. Richmond, UK, Kingprint.
- Singh B, Sahu PM, Singh AM (2002): Antimicrobial activity of pyrrolizidine alkaloids from Heliotropium subulatum.. Fitoterapia 73: 153–5. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Taha A, Al-Sayed H (2000): Brine shrimp bioassay of ethanol extracts of Sesuvium verrucosum, Salsola baryosma. and Zyqophyllum qatarense. medicinal plants from Bahrain. Phytother Res 14: 48–50. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Wojtaszek P (1997): Oxidative burst early plant repose to pathogen infection. Biochem J 322: 681–692. [PUBMED], [INFOTRIEVE]
- World Health Organization (1998): The world health report. Life in the 21st century: A vision for all, 2. Measuring health. Geneva, Switzerland, World Health Organization, pp. 39–60.
