ABSTRACT
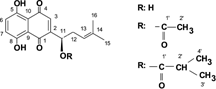
The phytochemical investigation of Lithospermum canescens. (Michx.) Lehm. roots with respect to pigment compounds resulted in the isolation of acetylshikonin and isobutyrylshikonin. Their structures were determined by NMR methods.
Introduction
Lithospermum canescens. (Michx.) Lehm. (Boraginaceae) (native American name: “hoary puccoon”) grows in open prairies in the northern United States and southern Canada. The native people use it as a body dye (Densmore, Citation1928). The occurrence of α-methylbutyrylshikonin and isovalerylshikonin is already reported (Wiedenfeld et al., Citation1998). Pigments of shikonin type are of importance because they are used for medical and cosmetic applications and show inflammatory, antibacterial, antifungal, immunostimulating, and anticancer activities. Furthermore, they stimulate the formation of tissue granulation (Couladouros et al., Citation1997; Sankawa et al., Citation1977Citation1981; Hisa et al., Citation1998; Pietrosiuk et al., Citation2003Citation2004aCitationb).
As Boraginaceae species show several pharmacological activities, we investigated Lithospermum canescens. for further active compounds. Previously, seven pyrrolizidine alkaloids have been isolated from this plant (lycopsamine, 7-O.-acetyllycopsamine, 7-O.-acetylintermedine, canescine, canescenine, acetylcanescine, and acetylcanescenine) (Wiedenfeld et al., Citation2003).
The aim of our current investigation was the isolation of further shikonin derivatives from mature plants of L. canescens..
Materials and Methods
Plant material
Plants of Lithospermum canescens. were collected at Parkland Bot. Togo, Saskatchewan, Canada. A voucher specimen of L. canescens. (Michx.) Lehm. from south of Togo, Saskatchewan, found in sandy soils, was deposited at The W.P. Fraser Herbarium (SASK), University of Saskatchewan, Saskatoon, Saskatchewan, Canada (accession no. 94815).
Extraction and isolation
Dried crumbled roots (200.0 g) of L. canescens. were extracted in Soxhlet apparatus for 72 h using n.-hexane. The solution was evaporated to dryness under reduced pressure, resulting in a deep red semisolid residue (5 g). The separation of the individual compounds was carried out using flash chromatography [stationary phase: Kieselgel 60; eluent: n.-hexane–methylene chloride (90:10 to 5:95), monitored at λ = 215 nm]. A final check of the isolated compounds was performed by High performance liquid chromatography (HPLC) (Dionex HPLC system equipped with automated sample injector (ASI-100) and UVD 340 S detector, Germany) under the following conditions: gradient elution acetonitrile (40–0 ml)/0.04 M orthophosphoric acid (60–100 ml); flow rate 1.5 mlmin−1; column: EC 250/4.6 Nucleosil 120–7 mm C18 (Macherey-Nagel, Germany).
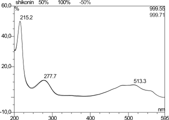
UV λmax(MeOH) for shikonin and its derivatives: 215, 278, 513 nm.
The 1H and 13C NMR spectra of the isolated compounds were measured in CDCl3 at 400 and 100 MHz, respectively, using a Bruker AC 400 spectrophotometer (Germany).
Results and Discussion
The separation of the pigment fraction from an n.-hexane extract of root material of L. canescens. led to the isolation of two compounds. UV spectral measurements showed the typical UV spectra of shikonin pigments (). The structures were determined by 1H and 13C NMR. Important data are found for C-6 (1H: 7.1 ppm/13C: 133 ppm), C-7 (1H: 7.1 ppm/13C: 133 ppm), and C-3 (1H: ∼ 7 ppm/13C: 131 ppm) as well as those for C-12 (1H: 2.4 ppm/13C: 33 ppm) and C-13 (1H: 5.1 ppm/13C: 118 ppm), which indicate the shikonin structure. The structure of the esterifying acid in 1 is demonstrated by the acetyl signals at ∼ 170 and 21 ppm (13C) and 2.12 ppm (1H) as well as the isobutyryl signals in 2 (19.0, 18.9, and 34.1 ppm = 13 C/1.15 and 2.6 ppm = 1 H). Complete data are presented in Tables and . These data prove the structures of 1 and 2 as acetyl and isobutyrylshikonin, respectively (). Our NMR data are in accordance with those found in the literature (Papageorgiou, Citation1980; Inoue et al., Citation1985; Man-Ho Cho et al., Citation1999). The HPLC results show that shikonin itself could not be detected in our plant material. Acetylshikonin was found to be the major pigment in the roots of L. canescens.. Our earlier studies (Wiedenfeld et al., Citation1998) of the upper material of the plant revealed the presence of two shikonin derivatives (α-methylbutyrylshikonin and isovalerylshikonin). Summarizing the results, it can be stated that the whole plant material of Lithospermum canescens. contains acetylshikonin, isobutyrylshikonin, α-methylbutyrylshikonin, and isovalerylshikonin. Free shikonin could not be detected.
Table 1.. 13C and 1H chemical shift data of acetylshikonin isolated from L. canescens..
Table 2. 13C and 1H chemical shift data of isobutyrylshikonin isolated from L. canescens..
Acknowledgments
We are thankful to Dr. Branka Barl, Saskatchewan Herb Research Centre; Department of Horticulture Science, University of Saskatchewan, Sakatoon, Canada, for collecting and identifying the plant material. We wish to thank Dr. Maria Niemyjska, Department of Organic Chemistry, Medical University of Warsaw, for helpful comments during the preparation of this paper. This work was supported by research grant PBZ-KBN-092/P05/2003 from the State Committee of Scientific Research.
References
- Couladouros EA, Plyta ZF, Strongilos AT, Papageorgiou VP (1997): Asymmetric synthesis of alkannin and shikonin. Tetrahedron Lett 38: 7263–7266.
- Densmore F (1928): Indian Use of Wild Plants for Crafts, Food, Medicine and Charms. Washington, DC, Smithsonian Institute, Bureau of American Ethnology, United States Government Printing Office, p. 290.
- Hisa T, Kimura Y, Takada K, Suzuki F, Takigawa M (1998): Shikonin, an ingredient of Lithospermum erythrorhizon., inhibits angiogenesis in vivo. and in vitro.. Anticancer Res 18: 783–790. [PUBMED], [INFOTRIEVE], [CSA]
- Inoue K, Akaji M, Inouye H(1985): Quinones and related compounds in higher plants. XXI. New finding on the proton and carbon-13 nuclear magnetic resonance spectra of shikonin. Chem Pharm Bull 33: 3993–3997.
- Man-Ho Cho, Young-Sook Paik, Tae-Ryong Hahn (1999): Physical stability of shikonin derivatives from the roots of Lithospermum erythrorhizon. cultivated in Korea. J Agric Food Chem 47: 4117–4120.
- Papageorgiou VP (1980): Carbon-13 NMR spectra of some naturally occurring hydroxynaphthoquinones. Planta Med 40: 305–307.
- Pietrosiuk A, Kędzia B, Hołderna-Kędzia E, Wiedenfeld H, Malinowski M, Furmanowa M (2003): Antimicrobial activity naphthoquinones from Lithospermum canescens. Lehm. Herba Polonica 49 (3/4): 209–215.
- Pietrosiuk A, Skopińska-Różewska E, Furmanowa M, Wiedenfeld H, Sommer E, Sokolnicka I, Bany J, Malinowski M (2004a): Immunomodulatory effect of shikonin derivatives isolated from Lithospermum canescens. on cellular and humoral immunity in Balb/c mice. Die Pharmazie 59(8): 640–642. [PUBMED], [INFOTRIEVE]
- Pietrosiuk A, Furmanowa M, Skopińska-Różewska E, Sommer E, Skurzak H, Bany J (2004b): The effect of acetyl shikonin isolated from Lithospermum canescens. roots on tumor-induced cutaneous angiogenesis. Acta Pol Pharm-Drug Res 61(5): 379–382.
- Sanakawa U, Ebizuka Y, Miyazaki T, Isomura Y, Otsuka H, Shibata S, Inomata M, Fukuoka F (1977): Antitumour activity of shikonin and its derivatives. Chem Pharm Bull 25: 2392–2395.
- Sankawa U, Otsuka H, Kataoka Y, Iitaka Y, Hoshi A, Kuretani K (1981): Antitumour activity of shikonin, alkannin and their derivatives. II. X-Ray analysis of cyclo-alkannin leucoacetate, tautomerism of alkannin and cyclo-alkannin and antitumour activity of alkannin derivatives. Chem Pharm Bull 29: 116–122. [PUBMED], [INFOTRIEVE]
- Wiedenfeld H, Pietrosiuk A, Furmanowa M, and Roeder E (1998): Pigment compounds in Lithospermum canescens. Lehm. Abstract of Plenary Lectures, Short Lectures and Posters of 46th Annual Congress Society for Plant Research, Quality of Medicinal Plants and Herbal Medicinal Products, University of Vienna, Vienna, Austria, p. G21.
- Wiedenfeld H, Pietrosiuk A, Furmanowa M, Roeder E (2003): Pyrrolizidine alkaloids from Lithospermum canescens. Lehm. Z Naturforsch 58c: 173–176.