ABSTRACT
A reversed-phase high-performance liquid chromatographic method for the determination of verbascoside and an analytical study of iridoids and others phenylpropanoids in Scrophularia scorodonia. L. is reported and validated. The method is applied for the analysis of stem, leaf, and flower extracts. The results showed that verbascoside represents 1.25% in leaf extracts.
Introduction
Polyphenolic compounds constitute one of the most characteristic classes of compounds in higher plants. Cinnamic acid derivatives occur widely in higher plants as esters or glycosides.
A number of reports have been published that demonstrate that phenylpropanoids posses a high potential of biological properties. Caffeic acid sugar esters have been shown to have antibacterial (Didry et al., Citation1999), antifungal, and antiviral activity (verbascoside and isoacteoside exhibited potent antiviral activity against respiratory syncytial virus) (Kernan et al., 1998), and inhibit 5-lipoxygenase (from the leucotriene biosynthesis), cyclic-AMP-phosphodiesterase, aldose reductase, and protein kinase C (Saracoglu et al., Citation1997). These compounds may also be in plants as protective agents and as repellent against herbivores (Ravn & Brimmer, Citation1988). Verbascoside and isoacteoside showed pronounced antihepatotoxic activity (Xiong et al., Citation1998). Verbascoside and angorosides A, B, and C were found to exhibit activity against several kinds of cancer cells (Pettit et al., Citation1990; Saracoglu et al., Citation1997). Verbascoside, isolated from Scrophularia scorodonia., has shown antiviral activity against vesicular stomatitis virus (Bermejo et al., Citation2002).
The genus Scrophularia. is known to contain a variety of phenolic compounds. It has been suggested that the therapeutic action depends on the presence of phenylpropanoid glycosides. On the other hand, different species of the Scrophularia. genus (Scrophulariaceae) have been reported to have bacteriostatic and anti-inflammatory properties (Fernández et al., Citation1998). Phenylpropanoids isolated from Scrophularia buergeriana. miq. may exert significant protective effects against glutamate-induced neurodegeneration in primary cultures of cortical neurons (So & Young, Citation2000).
In the course of a search for biologically active substances from Spanish plants, the phytochemical analysis of Scrophularia scorodonia. L. revealed that it is a rich source of polyphenols of therapeutic interest, and a part of the possible biological activity of this species could be due to polyphenolic compounds.
Materials and Methods
Scrophularia scorodonia. was collected from Jaén (Spain) in June 2000. The plant material was separated into flowers, stems, and leaves. The voucher specimen (SS 2000) was deposited at the Laboratory of Pharmacognosy, University of Alcalá, Madrid. Authentic samples of pure verbascoside were isolated from S. scorodonia. in our laboratory in earlier studies.
High-performance liquid chromatography (HPLC) analysis was carried out with a Hewlett Packard Series 1100 chromatograph (USA) with a photodiode-array detector at λ = 278 nm. A prepacked analytical column (300 × 3.9 mm) [Phenomenex Bondclone Interchim (Montluçon, France) C18 column] was used with a μ-Bondapak C18 guard column (Waters, USA). The eluents were water from pump A and methanol from pump B according to . A step gradient was used to allow a better separation of the main phenylpropanoids of this species; the HPLC analysis is simple and can easily be automated. The flow rate was kept constant at 1.5 ml/min.
Table 1.. Gradient table.
Ten grams of leaves, stems, and flowers were extracted with 100 ml of 80% MeOH for 12 h. Aliquots of 20 µl of these extracts were used for analyses. The standard compound (verbascoside) was dissolved in HPLC-grade methanol.
The quantitative determination was carried out using external standard for calibration. The linearity of the HPLC method was observed in the range of 0.4–1.3 mg/ml. Data for least-squares regression analysis of the calibration graphs were y. = 430.74 + 191.37x. (r. = 0.98), where y. = peak area, x. = concentration in mg/ml, and r = correlation coefficient. The reproductibility of the method was calculated by assaying 10 replicates of the same 80% MeOH extract. The relative standard deviation for flower extracts was estimated to be 1.5%. The reproductibility of verbascoside standard preparation was tested by assaying 10 preparations at a concentration of 0.7 mg/ml. The relative standard deviation was estimated to be 1.39%. Four stem extractions with 80% MeOH were tested for the reproductibility of the extraction method. The relative standard deviation was 3.85%. At a 2:1 signal-to-noise ratio, the limit of detection of verbascoside was determined to be 0.5 µg/ml. Quantification limit was 200 µg/ml for verbascoside, on 10 injections; the coefficient of variation was 2.18%.
Results
Concentration of samples was determined directly from the calibration graphs. The amount of verbascoside in leaves and flowers is very important, and it is more concentrated in leaves. Quantitative analysis of 80% MeOH extracts showed that the amount of verbascoside is 0.76% in flower extracts, 1.25% in leaf extracts, and 0.58% in stem extracts. In a previous paper we have reported that S. scorodonia. is a potential new source of harpagoside (Díaz et al., Citation1998). Althought this species has not been used in folk medicine, the presence of verbascoside and harpagoside, which have showed important pharmacological activities, increases the possible pharmacological value of this species.
So, for the first time, this method led to a better control of these important active principles in the extracts of S. scorodonia..
Discussion
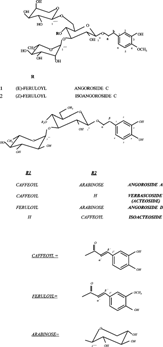
We have previously reported a reversed-phase high-perfomance liquid chromatographic method for the determination of harpagoside, one of the main biologically active constituents, according to current pharmacological knowledge, in S. scorodonia. (Díaz et al., Citation1998). Seasonal variations on the content of harpagoside in this species were also investigated (De Santos et al., Citation2000a). Later, we reported the isolation and structure elucidation of different phenylpropanoids glycosides from S. scorodonia.: angoroside A, angoroside C, isoangoroside C, verbascoside, and isoacteoside (Fig.) (De Santos et al., Citation2000bCitationc). The method used for the quantitative analysis of harpagoside showed good resolution of iridoids. Later, we observed that the method was not appropriate for the separation of phenylpropanoid in different extracts of this species, because the very complex phenylpropanid composition of this species requires further improvement and development of another HPLC procedure. So, the reversed-phase high-perfomance procedure reported in this paper is more promising because it allows high resolution and a rapid and reproducible determination of the phenylpropanoids in S. scorodonia.. So, we have modified the method proposed for quantitative analysis of harpagoside to obtain a complete separation of the main phenylpropanoids and iridoids of this species that could be responsible for the pharmaceutical properties of S. scorodonia.
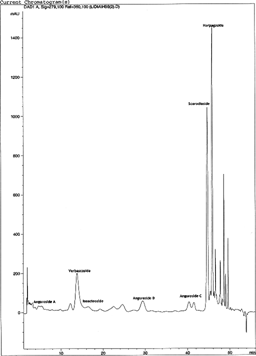
This HPLC method was found to be more complete and showed a better resolution of phenylpropanoids from different methanol extracts of Scrophularia scorodonia.. Two iridoids (scorodioside and harpagoside) and five phenylpropanoids glycosides (verbascoside, isoacteoside, and angorosides A, C, and D) have been identified based on the comparison of retention time with our isolated compounds. A typical chromatogram of 80% MeOH leaf extract is shown in .
References
- Bermejo P, Abad MJ, Díaz AM, Fernández L, De Santos J, Sánchez S, Villaescusa L, Carrasco L, Irurzun A (2002): Antiviral activity of seven iridoids, three saikosaponins and one phenylpropanoid glycoside extracted from Bupleurum rigidum. and Scrophularia scorodonia.. Planta Med 68: 106–110. [PUBMED], [INFOTRIEVE], [CSA]
- De Santos J, Fernández L, Díaz AM, Villaescusa L (2000a): Seasonal variations in the harpagoside content of Scrophularia scorodonia. L. Z. Naturforsch. C 55c: 1035–1037.
- De Santos J, Díaz AM, Fernández L, Rumbero A (2000b): A new phenylpropanoid glycoside isolated from Scrophularia scorodonia. L. Magn Reson Chem 38: 688–691. [CROSSREF]
- De Santos J, Díaz AM, Fernández L, Rumbero A (2000c): Isoangoroside C, a phenylpropanoid glycoside from Scrophularia scorodonia. roots. Z Naturforsch 55c: 333–336.
- Díaz A, Fernández L, Ollivier E, Martín T, Villaescusa L, Balansard G (1998): Reverse-phase high pressure liquid chromatography analysis of harpagoside, scorodioside and verbascoside from Scrophularia scorodonia.: Quantitative determination of harpagoside. Planta Med 64: 94–95.
- Didry N, Seidel V, Dubreuil L, Tillequin F, Bailleul F (1999): Isolation and antibacterial activity of phenylpropanoids derivates from Ballota nigra.. J Ethnopharmacol 67: 197–202. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Fernández MA, Saenz MT, García MD (1998): Antiinflammatory activity in rats and mice of phenolic acids isolated from Scrophularia frutescens.. J Pharm Pharmacol 50: 1183–1186.
- Kernan MR, Amarquaye A, Chen JL, Chan J, Sesin DF, Parkinson N, Ye ZJ, Barret M, Bales C, Stoddart CA, Sloan B, Blanc P, Limbach C, Mrisho S, Rozhon EJ (1988): Antiviral phenylpropanoid glycosides from the medicinal plant Markhamia lutea.. J Nat Prod 61: 564–570. [CSA], [CROSSREF]
- Pettit GR, Numata A, Takemura T, Ode RH, Narula AS, Schmidt JM, Cragg GM, Pase CP (1990): Antineoplasic agents, 107. Isolation of acteoside and isoacteoside from Castilleja linariaefolia... J Nat Prod 53: 456–458. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Ravn H, Brimmer L (1988): Structure and antibacterial activity of plantamajoside, a caffeic acid sugar ester from Plantago major. subs. major.. Phytochemistry 27: 3433–3437. [CSA], [CROSSREF]
- So RK, Young CK (2000): Neuroprotective phenylpropanoid esters of rhamnose isolated from roots of Scrophularia buergeriana.. Phytochemistry 54: 503–509. [CSA], [CROSSREF]
- Saracoglu I, Calis I, Inoue M, Ogihara Y (1997): Selective cytotoxic and cytostatic activity of some phenylpropanoid glycosides. Fitoterapia 68: 434–438.
- Xiong Q, Hase K, Tezuka Y, Tani T, Namba T, Kadota S (1998): Hepatoprotective activity of phenylethanoids from Cistanche deserticola.. Planta Med 64: 120–125. [PUBMED], [INFOTRIEVE], [CSA]