Abstract
The soft coral genus Sinularia. is one of the most widely distributed soft corals. It constitutes a dominant portion of the biomass in the tropical reef environment. Sinularia. elaborates a rich harvest of secondary metabolites including sesquiterpenes, diterpenes, polyhydroxylated steroids, and polyamine compounds. These metabolites were recently shown to possess a range of biological activities such as antimicrobial, anti-inflammatory, and cytotoxic activities. During the past decade, Sinularia. has yielded many new structures with novel skeletons. Several of the previously published secondary metabolites have been reexamined for their pharmacological properties, and the results strongly support further investigations. The current article reviews the terpenoids of the soft coral genus Sinularia. and their pharmacological significance.
Introduction
In recent years, the current status of marine natural product bioprospecting has been a popular subject for reviews (Kelecom, Citation1999; Proksch et al., Citation2002; Alejandro & Gustafson Citation2003; Haefner, Citation2003), as it has resulted in a considerable number of drug candidates. The study of marine natural product chemistry began in 1960 with a conference entitled “Biochemistry and Pharmacology Derived from Marine Organisms” organized by the New York Academy of Sciences. Beginning in 1978, Scheuer (Citation1978) and Faulkner (Citation2002) provided comprehensive reviews on the chemical and biological perspectives of marine natural products and, therefore, played a central role in shaping the field of marine natural product chemistry.
Coral reefs represent an extraordinary diverse biota in tropical environments, and soft corals often constitute a dominant part of the reef biomass. Soft corals attracted considerable attention because of the wide range of bioactive secondary metabolites generated by these marine invertebrates. The interest began with the isolation of the prostaglandin [(15R.)-PGA2] from the gorgonian Plexaura homomalla. Esper, 1792 (Weiheimer & Spraggins, Citation1969). This was followed, in the early 1970s, with the examination of the Alcyonacean octocorals. Chemists came to the conclusion that terpenoid chemistry predominates across the Octocorallia and Tursch et al. (1978) surveyed the known distribution of terpenoids across the Octocorallia. Coll (Citation1992) reviewed the chemistry and chemical ecology of Octocorals and made several contributions to the ecological reasons for the structural chemical diversity. More recently, Venkateswarlu et al. (Citation2001) gave an account on the novel bioactive metabolites from soft corals.
Sinularia. is a soft coral belonging to the phylum Cnidaria, class Alcyonaria, and family Alcyoniidae. Its taxonomic identification is largely based on the examination of the spicules, and a fairly reliable taxonomic key is available to guide species identification (Verseveldt, Citation1980). The genus Sinularia. consists of almost 90 species of which more than 50 have been chemically examined. Many bioactive metabolites that have been reported from soft corals and species of the genus Sinularia. have been studied, and the isolated components display potential bioactivities such as antimicrobial, anti-inflammatory, and cytotoxic activities (Venkateswarlu et al., Citation2001).
The study of Sinularia. has been dominated by Australian, Indian, and Japanese groups who have described a wide range of secondary metabolites including sesquiterpenes, diterpenes, polyhydroxylated steroids, and polyamine compounds. A number of questions were raised in the past regarding the origin of terpenes in symbiotic associations between marine invertebrates and algae (Zooxanthellae). Although Kobbe et al. (Citation1984) came to the conclusion that the zooxanthellae do not make terpenes and that the coral polyp was the active organelle in terpenoid biosynthesis, the subject is still in question. Anjaneyulu and Venkateswalu (Citation1995) reviewed the chemical components of the soft coral genus Sinularia.. Since 1995, the number of research papers investigating the chemical constituents of the soft coral genus Sinularia. has exceeded 100, the majority reporting new and novel terpenoids. In addition, many of the previously published terpenoids were reexamined for their pharmacological activities, and the results strongly promoted further investigations. The purpose of this review is to focus on the terpenoid constituents of Sinularia., highlighting their novel chemistry, pharmacological activities, and biomedical applications, and to examine the future perspectives for soft coral research.
Sesquiterpenes
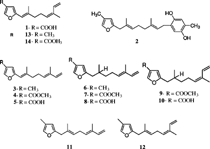
The first sesquiterpene reported from the genus Sinularia. was a furano-sesquiterpenoid acid (1) () isolated from S. gonatodes. Ehrenberg, 1834 by an Australian group (Coll et al., Citation1977). This was followed by a new furanoquinol (2) isolated from S. lochomodes. Kolonko, 1926 (Coll et al., Citation1978). Twelve related sesquiterpene furans (3–14) were subsequently reported from two Sinularia. species, S. capillosa. Tixier-Durivault, 1970, and S. firma. Tixier-Durivault, 1970 (Bowden et al., Citation1983). In 1994, the furanoic acid (1) was found to inactivate bee venom phospholipase A2 (bvPLA2) in vitro. with an IC50 of 0.5 µM (Grace et al., Citation1994). The study of its kinetics of inactivation suggested that it may interact with multiple binding sites on bvPLA2. Phospholipase A2 (PLA2), which is involved in the pathogenesis of a variety of inflammatory diseases, is a promising target for the development of anti-inflammatory drugs. Intrigued by the anti-inflammatory activity of this simple compound, Williams and Faulkner (Citation1996) synthesized its geometrical isomer (5), which inhibited bee venom PLA2 (46% at 0.8 µg/ml). They-designed two practical syntheses, which were both based on Claisen rearrangements. The geometrical isomeric compounds 7 and 9 showed a minimum inhibitory concentration (MIC) of 32 µg/ml against Mycobaterium tuberculosis. Lehman & Neumann, 1896 (TB), when combined (Kamel et al., unpublished data).
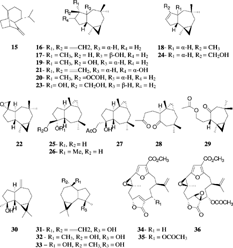
A sesquiterpene (15) having a non-farnesolic skeleton (i.e., cannot be derived by direct cyclization of a farnesol precursor) was isolated from S. mayi. Luttschwager, 1914, in 1978 and named sinularene (Beecham et al., Citation1978aCitationb). Having a unique skeleton, sinularene has attracted considerable attention as a synthetic target, and several total syntheses of sinularene have been reported using different approaches (Collins & Wege Citation1979; Oppolzer et al., Citation1982Citation1984; Antczak et al., Citation1987) such as intramolecular Diels-Alder reaction (Antczak et al., Citation1987) and intramolecular magnesium-ene reaction as the key steps (Oppolzer et al., Citation1982Citation1984).
The most commonly reported sesquiterpene from the genus Sinularia. is Δ9,(15) africanene (16). It is a tricylic sesquiterpene belonging to the rare africanane skeleton, which was first found in africanol (17), a tertiary alcohol isolated from the octocoral Lemnalia africana. Gray, 1868 (Tursch et al., Citation1974). Africanene (16) was first isolated by Braekman et al. (Citation1980) and Kashman et al. (Citation1980) independently from S. polydactyla. Ehrenberg, 1834, and S. erecta. Tixier-Durivault, 1945, respectively. The absolute configuration was assigned by an X-ray diffraction study of derivatives. Recently, the pharmacological activities of africanene have been investigated (Reddy et al., Citation1999). It showed mild CNS depressant activity and dose-dependent hypotensive activity (). Furthermore, it exhibited anti-inflammatory activity against carrageenan-induced rat edema with a 60% reduction at a dose of 10 mg/kg body weight as compared to ibuprofen, which showed 49% reduction at a higher dose of 100 mg/kg body weight orally (Reddy et al., Citation1999). This anti-inflammatory effects suggests that it is worthy of further characterization studies.
Table 1.. Biological activities of terpenoids from the soft coral genus Sinularia..
Moreover, africanene exhibited cytotoxic activity against the Ehrlich ascites carcinoma (EAS) and Dalton's lymphoma ascites tumor (DLAT) cells. In an in vivo. cytotoxic assay, africanene increased the life span of mice with EAS by 22% and 53% at the concentrations of 10 and 20 mg/kg body weight, respectively (Reddy et al., Citation1999). Chemical and biocatalytic structure activity relationship (SAR) studies have been initiated for africanene (Venkasterwarlu et al., Citation1999a; Reddy et al., Citation2000). Δ9,10-Africanene (18), leptographiol (9β-hydroxy africanane) (19), and O.-formyl leptographiol (O.-formyl 9β-hydroxy africanane) (20) were obtained by means of acid catalyzed rearrangement of africanene (Reddy et al., Citation2000), and 10α-hydroxy-9(15)-africanene (21) and 9α,15-epoxyafricanane (22) were obtained by microbial oxidation of africanene using Aspergillus niger. Teigh, 1867, and Rhizopus oryzae. Went. & Prinns. Geerl., 1895 (Venkateswarlu et al., Citation1999a). Compounds 21 and 22 were reported earlier as natural products along with a new oxygenated derivative, 9α,15-dihydroxyafricanane (23) from S. dissecta. Tixier-Durivault, 1945 (Ramesh et al., Citation1999). Later, the same group reported 15-hydroxy-Δ9-africanene (24) from this species (Ramesh et al., Citation2001). Sinularia intacta. Tixier-Durivault, 1945, of the Indian ocean has yielded three new derivatives, 9(R.)-africanane-9,15-diol (25), 9(R.)-9-methoxyafricanan-15-ol (26), and 9(S.)-africanane-9,15-diol-15-monoacetate (27) (Anjaneyulu et al., Citation1999). Recently, several secoafricanene derivatives (with the five membered-ring cleaved) have been isolated from different Sinularia. species. The diketosesquiterpenoid 8,9-secoafricanene-8,9-dione (28) was reported from S. intacta. Tixier-Durivault, 1945, of the Indian Ocean (Anjaneyulu & Gowri, Citation1999), and 4-acetoxy-3,15-dinor-2,3-seco-2-africanone (29) was reported from S. dissecta. of the Mandapam coast (Ramesh & Venkateswarlu, Citation2000). None of these africanene derivatives has been pharmacologically evaluated. However, the pharmacological activities reported for africanene are promising and encourage further investigations, and the biological testing of its derivatives should yield an optimized drug lead and future SAR.
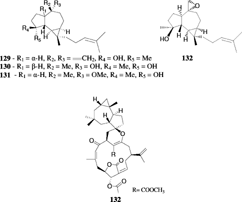
Recently, spathulenol (30), a rare sesquiterpene with an aromadendrane skeleton found in the plant Eucalyptus spathulata. Hook, 1844, was isolated from S. kavarattiensis. Aldersalde & Shirwaiker, 1991 (Goud et al., Citation2002). Aromadendrene (31), 4α,7β-aromadendranediol (32), and 4α,7α-aromadendranediol (33) were identified from S. mayi. in 1978 (Beechan et al., Citation1978b). Several aromadendrane diterpenoids have been isolated from Sinularia. species (see next sections).
Cembranoid diterpenes
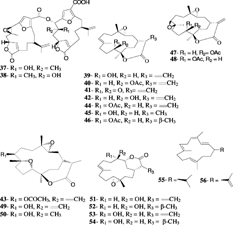
Cembranoids are a class of diterpenes possessing a 14-membered ring skeleton. Pukalide (34) was first isolated from S. abrupta. Tixier-Durivault, 1970, in 1975 by the Scheuer group (Missakian et al., Citation1975). The derivatives 13α-acetoxy pukalide (35) and 13α-acetoxy-11β,12β-epoxypukalide (36) were later obtained from an Australian S. polydactyla. Ehrenberg, 1834 (Bowden et al., Citation1989). Pukalide and its derivatives are produced by a variety of octocorals in the orders Gorgonacea and Alcyonacea. They are structurally similar to the gorgonian-derived compound lophotoxin and to bipinnatins, both of which are irreversible nicotinic acetylcholine receptor antagonists (Abramson et al., Citation1991a). In a structure-activity study of the lophotoxin family, 13α-acetoxypukalide (35) was found to be active (Abramson et al., Citation1991b). This study defined the C7-C8 epoxide and the acetate ester at C13 to be essential for the activity. The interaction of cembranoids with the acetylcholine receptors was characterized earlier. It was shown that the irreversible inhibition results from a specific covalent reaction between the cembranoid and Tyr190 in the α-subunit of the receptor. Recently, two bis.-pukalide diterpenes were reported from S. erecta. Tixier-Durivault, 1945, named mayotolides A and B (37, 38) (Rudi et al., Citation1998). Pukalide, having a furanocembranolide skeleton, has been a challenge to synthesize. Generally, constructing the furanocyclized structure that typifies many of these compounds has been a problem. Although a total synthesis has not been reported to date, recently the synthesis of the C1-C18 segment of pukalide was accomplished in 11 steps with a 10% overall yield (Peter & Michael, Citation2002).
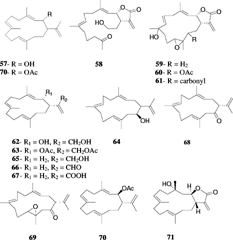
Sinulariolide (39) was first isolated from S. flexibilis. Quoy & Gaimard, 1833 (Tursch et al., Citation1975), and was later reported from other Sinularia. species. The structure was determined by spectroscopic and chemical methods, and the absolute configuration was established by X-ray diffraction by Karlsson (Citation1977). Sinulariolide (39) demonstrates a broad range of biological activities, having been reported as an algicidal molecule responsible for antifouling properties (Tursch, Citation1976) and showing marginal cytotoxic activities against a number of cell lines (Sui-jian et al., Citation2002). Several sinulariolide derivatives were later reported including 11-episinulariolide acetate (40) and 11-dehydrosinulariolide (41) from Red Sea species (Kashman et al., Citation1977) and 11-epi-sinulariolide (42) and 11-epoxysinulariolide (43) from S. flexibilis. Quoy & Gimard, 1833 (Mori et al., Citation1983). A series of analogues (44–50) were prepared from sinulariolide and 11-epi-sinulariolide acetate, and their cytotoxic activities were evaluated (Hsieh et al., Citation2003). It was shown that the bioactivities diminish in compounds with hydrogenated α-methylene-lactone system, epoxidized double bond C-7, 8, and/or an ether linkage between C-8 and C-11.
Flexibilide (51) and dihydroflexibilide (52) were reported from the Australian species of S. flexibilis. (Kazlauskao et al., Citation1978). Flexibilide was reported to possess anti-inflammatory and antiarthritic activities in rats (Buckle et al., Citation1980). It reduced paw edema induced by carrageenan with a similar potency to phenylbutazone, and both (51 and 52) showed cytotoxic activities in vitro. (Sui-jian et al., Citation2002). Flexibilide, dihydroflexibilide, and sinulariolide were shown to be cardioactive, producing vasorelaxant responses in the isolated rat tissues (Aceret et al., Citation1996). Flexibilide and sinulariolide also exhibited antimicrobial activity and inhibited growth of Gram-positive bacteria Bacillus subtilis. Cohn, 1872, and Staphylococcus aureus. Rosenbach, 1884 (Aceret et al., Citation1998). It was speculated that the antibacterial activity is associated with the α-methylene-lactone moiety present in flexibilide and sinulariolide, but not in dihydroflexibilide. The cytotoxic cembranolides sinularin (53) and dihydrosinularin (54) were isolated from the same species, and their structures and absolute configurations were determined by X-ray diffraction analysis (Weinheimer et al., Citation1977). Dihydrosinularin and 11-episinulariolide were both isolated from the marine mollusk Planaxis sulcatus. Born, 1778, and it was suggested that they were of dietary origin in the mollusk (Sanduja et al., Citation1986).
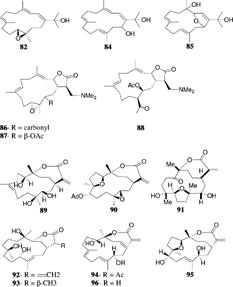
Sinularia mayi. Luttschwager, 1914, is a common species in the coral reefs of southern Japan and has been subjected to intensive chemical studies by Japanese groups. The parent hydrocarbon cembrene (55) was reported from S. mayi. along with cembrenene (56) and the monooxycembratetraene mayol (57) (Uchio et al., Citation1981). Later, the first secocembrane type diterpenoid, mayolide A (58), was reported from the same species (Kobayashi, Citation1988). A secocembranoid has a unique skeleton in which the bond between the C-12 and C-13 positions of the 14-membered carbocyclic skeleton of usually encountered cembranolides is cleaved. The cembranoids mayolide B, C and D (59–61) were also reported from S. mayi. Luttschwager, 1914. The stereoselective total synthesis of mayolide A has been achieved starting from d-mannitol (Nagaoka et al., Citation1992). Sinularia mayi. also yielded sinulariol A–D (62–65), sinularial A (66), sinularic acid A (67), and sinularones A and B (68, 69) (Kobayashi et al., Citation1987; Kobayashi & Hamayuchi, Citation1988). Sinulariol B has been synthesized from E.-geraniol with a ∼ 10% overall yield (Lan et al., Citation1999; Yue et al., Citation1999). Mayolacetate (70) and a hydroxycembranolide γ.-lactone (71) were obtained from S. hirta. Pratt, 1903 (Anjaneyulu et al., Citation1996).
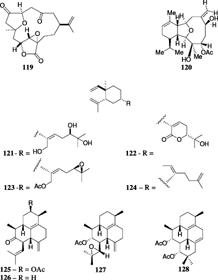
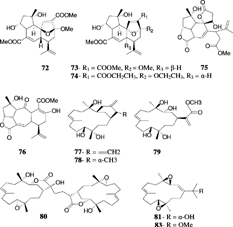
Mandapamate (72) is a diterpene related to the cembranoid series but with a more complex tetracyclic carbon skeleton and has been isolated from S. dissecta. Tixier-Duricvault, 1945 (Venkateswarlu et al., Citation1994). The diastereomeric, isomandapamate (73), was reported from S. maxima. Verseveldt, 1970 (Anjaneyulu et al., Citation1995b), and its homologue, bishomoisomandapamate (74), was reported from an undescribed Sinularia. sp. (Anjaneyulu & Sarada, Citation1999). It was postulated that the tetracylic framework of mandapamate could be derived by an intramolecular Diels-Alder reaction. Anjaneyulu et al. (Citation2000) provided a possible biogenetic sequence for mandapamate and havellockate (75), which represent a novel seco. and spiro. lactone diterpenoid reported from S. granosa. Tixier-Durivault, 1970, of the Indian Ocean (Anjaneyulu et al., Citation1998). The structures were determined by a detailed study of their spectral data including X-ray analysis (Anjaneyulu et al., Citation1998). A related diterpene with an unusual 5-7-6 tricyclic skeleton, rameswaralide (76), was isolated from S. dissecta. (Ramesh et al., Citation1998).
Bioactivity guided fractionation of the extract of S. flexibilis. yielded sinuflexolide (77), dihydrosinuflexolide (78), and sinuflexibilin (79) (Duh et al., Citation1998b). Compounds 77 and 79 exhibited significant cytotoxicity. A biscembranoid diterpene, sinuflexin (80), was reported from S. flexibilis. (Duh et al., Citation1998a) and showed cytotxicity against the P-388 cell culture system. Sinugibberol (81) is a cytotoxic cembranoid isolated from S. gibberosa. Tixier-Durivault, 1970 (Hou et al., Citation1995). The same species yielded four new cytotoxic cembranoids: 11,12-epoxy-1(E.),3(E.),7(E.)-cembratrien-15-ol (82), 3,4:11,12-diepoxy-15-methoxy-1(E.),7(E.)-cembradiene (83), 1(E.),3(E.),7(E.),11(E.)-cembratetraene-14,15-diol (84), and 3,14-epoxy-1(E.),7(E.),11(E.)-cembranetriene-4,15-diol (85) (Duh & Hou, Citation1996).
Three nitrogen-containing cembranolide diterpenes, sinulamine I (85), II (86), and III (87), each possessing a dimethylamino group, were isolated from an Okinawan species, and their structures were elucidated by spectroscopic analysis and chemical transformations (Iguchi et al., Citation1992). Later, Kobayashi and Ishizaka (Citation1992) revised the stereochemistry of these dimethylamine adducts and concluded that they should be regarded as formed in the organism during storage. A similar diterpene with a dimethylamino group isolated from the soft coral Lobopytum. exhibited moderate HIV-inhibitory activity (Rashid et al., Citation2000).
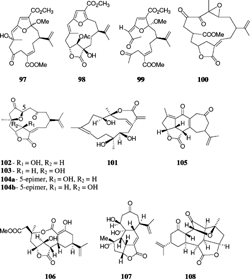
Capillolide (89) was isolated from S. capillosa. along with flexibilide, sinulariolide, and (1R.,13S.,12S.,9S.,8R.,5S.,4R.)-9-acetoxy-5,8:12,13-diepoxycembr-15(17)-en-16,4-olide (90) (Su et al., Citation2000). Capillolide showed moderate cytotoxic activity. Sinulariolone (91), a new highly oxygenated cembranoid, was obtained from a Philippine collection of S. flexibilis. (Guerrero et al., Citation1995), and the trihydroxy cembranolide lactones flexibiolide (92) and dihydroflexibiolide (93) were isolated from an Indian collection of S. flexibilis. (Anjaneyulu & Sagar, Citation1996). This same species has yielded new cembranoilde €-lactones named sandensolide monoacetate (94) and flexibolide (95) (Anjaneyulu et al., Citation1997a). Sandensolide (96) was isolated from S. sandensis. Verseveldt, 1977, of the Indian Ocean (Anjaneyulu et al., Citation1995a). Sinularia maxima. Verseveldt yielded the new furanocembranoid derivatives named sethukarailin and sethukarailide (97, 98) (Venkateswarlu et al., Citation1999b). Seco-sethukarailin (99) was recently obtained from another species, S. dissecta. (Reddy et al., Citation2002). Sinulariadione (100), an uncommon 1,3-diketone cembranoid, was isolated from an unidentified Sinularia. from the Mandapam coast (Anjaneyulu & Raju, Citation1995). More recently, the crystal structure of a new cytotoxic cembranolide diterpene (101) was reported from S. tenella. Li, 1982, by a Chinese group (Lin et al., Citation2002).
Norcembranoid diterpenes
The first norcembranolide (102) (which lacks a C-18 carbon atom at C-4) was isolated from S. leptoclados. Ehrenberg, 1834, by an Australian group (Bowden et al., Citation1978) and was reported later from several other species. The relative stereochemistry, derived by NMR spectroscopic data, was confirmed by X-ray diffraction analysis (Turner et al., Citation1979). However, the structure was drawn in error showing the configuration at C-11 opposite to what had been established by the crystal structure (Turner et al., Citation1979). Misinterpretation of the X-ray data and of the stereochemistry at C-11 has led to confusion in the literature, and this error has been propagated in many of the later papers (Lakshmi & Schmitz, Citation1986; Duh et al., Citation1993) and reviews (Wahlberg & Eklund, Citation1992) and is still repeated as late as 2003 (Takaki et al., Citation2003). Sato et al. (Citation1985) later reported the C-11 epimer (103) while Shoji et al. (Citation1993) reported the C-5 epimer (104a) and named it sinuleptolide, and Ramesh and Venkateswarlu (Citation2000) reported 11-epi sinuleptolide (104b). Compounds 102 and 103 showed cytotoxic activities (Sheu et al., Citation2002), and compounds 102 and 104a showed an inhibitory effect on LPS-induced TNF-α production (Takaki et al., Citation2003).
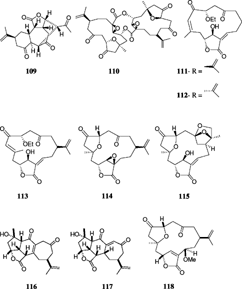
Several other norcembrane diterpenes have been isolated from different Sinularia. species. Yonarolide (105) and sinulariadiolide (106) were both isolated from an Okinawan Sinularia.. Yonarolide possesses a novel tricyclo [7.5.0.03,7]-tetradecane skeleton (Iguchi et al., Citation1995), whereas sinulariadiolide is characterized by a new carbon skeleton with two lactone moieties, five- and nine-membered rings; the nine-membered system constitutes a β-hydroxy-α, β-unsaturated ester group (Iguchi et al., Citation1996). Dissectolide (107), isolated from the Indian Ocean S. dissecta., is shown to have a highly strained tetracyclic ring system with cyclopentane, cyclohexene, cycloheptane, and α,β-unsaturated γ.-lactone ring systems (Kobayashi et al., Citation1995). The norditerpenoid ineleganolide (108) was isolated from a Formosan S. inelegans. Tixier-Durivault, 1970, and exhibited moderate cytotoxicity (Duh et al., Citation1999). Horiolide (109) was isolated from a Sinularia. species and was shown to possess a new carbon skeleton having one six-membered cyclohexane ring bearing an isopropylene moiety, a carbonyl group, and one seven-membered ring attached to a five-membered lactone moiety (Radhika et al., Citation2002). The Red Sea S. gardineri. Pratt, 1093, yielded a novel cytotoxic cembranoid dimer, singardin (110), which was identified as a dimer of 5-epi-sinuleptolide and sinuleptolide with an epoxide replacing the Δ12 olefin (Elsayed & Hamann, Citation1996). Sinularia leptoclados. and S. parva. Tixier-Durivault, 1970, yielded five new norcembranoids, leptocladolides A–C (111, 114, and 115), 1-epi.-leptocladolide (112), and 7E-leptocladolide (113) (Ahmed et al., Citation2003) with 114 and 115 exhibiting cytotoxic activities. Scabrolides A–D (116–119) were isolated from a Taiwanese S. scarab. Tixier-Durivault, 1970 (Sheu et al., Citation2002).
Non-cembranoid diterpenes
The first non-cembrane based diterpene, alcyonin (120), had a cladiellane skeleton and was isolated from an Okinawan species of S. flexibilis. (Kusumi et al., Citation1988). The same species was later reported to yield three lobane diterpenes (121–123) (Hamada et al., Citation1992). Recently, a cytotoxic lobane diterpene, ineleganene (124), was isolated from the Formosan S. inelegans. and showed cytotoxic activity (Chai et al., Citation2000). The amphilectane-type diterpenoids, Sinulobatins A–D (125–128), were reported from the Japanese S. nanolobata. Verseveldt, 1977 (Yamada et al., Citation1997), and exhibited cytotoxicity. The first report of aromadendrane diterpenoids (129–132) in marine systems was made from a new species of Sinularia. of the Indian Ocean whose extract showed moderate larvicidal activity (Anjaneyulu et al., Citation1997b).
Unusual chemistry of a Sinularia. hybrid
Polymaxenolide (133) is a novel metabolite isolated from the hybrid soft coral Sinularia maxima. x S. polydactyla. (Kamel et al., Citation2004). It is composed of a cembrane-africanane joined skeleton via a C,C-linkage. It was hypothesized that polymaxenolide represents a new mechanism of qualitative variation in hybrid secondary chemistry. It is formed by the combination of the basic skeleton of one parent (africanane sesquiterpene) with a basic skeleton from the other parental species (cembrane diterpene). To date, no bioactivity has been reported for this compound.
Conclusions
Marine natural products chemistry, and in particular, Alcyonaria chemistry has had a major impact on drug discovery and development over the past 25 years and will continue to inspire the chemistry of the future. The biological and pharmacological properties associated with soft coral terpenoids have only recently been investigated but are shown to be highly promising and support further investigations.
Cembranoid diterpenes isolated from soft corals are known to exhibit cytotoxic and antitumor properties, and future studies investigating the differential activity of these metabolites as well as their mechanism of action will be extremely valuable. The limited data available support the need for more extensive structure-activity relationship studies of cembranoid diterpenes in order to develop lead anticancer compounds. In addition, the anti-inflammatory properties of some Sinularia. metabolites such as africanene and flexibilide require a further evaluation of the mechanism of action. The reasonable yield of these natural products makes them intriguing for semisynthetic and biocatalytic modifications aimed at the reduction of toxicity while enhancing the activity. However, this is not always the case; because one major reason for the absence of biological data of Sinularia. terpenoids in the literature is the low yield. In addition, many researchers do not have access to relevant bioassays.
The discovery of a novel metabolite, polymaxenolide, from a hybrid coral represents an intriguing piece of data that implicates that new gene hybrids have the potential to generate new interesting molecules. Future research should investigate coral hybrid chemistry.
Acknowledgments
The authors are grateful to Dr. Mark Hamann for his thorough reading of the manuscript and Dr. Frank Fronszek for his helpful discussion.
References
- Abramson SN, Fenical W, Taylor P. (1991a) Lophotoxins: Irreversible active-site-directed inhibitors of nicotinic acetylcholine receptors. Drug Dev Res 24: 297–312.
- Abramson SN, Trischman JA, Edward DM, Harold EE, Fenical W, Taylor P (1991b): Structure activity and molecular modeling studies of lophotoxin family of irreversible nicotinic receptor antagonists. J Med Chem 34: 1789–1804.
- Aceret TL, Brown L, Miller J, Coll JC, Sammarco PW (1996): Cardiac and vascular responses of isolated rat tissues treated with diterpenes from Sinularia flexibilis.. Toxicon 34: 1167–1171.
- Aceret TL, Coll JC, Uchio Y, Sammarco PW (1998): Antimicrobial activity of the diterpenes flexibilide and sinulariolide derived from Sinularia flexibilis. Quoy and Gaimard. Comp Biochem Physiol (C) 120: 121–126.
- Ahmed AF, Shiue R, Wang G, Dai C, Kuo Y, Sheu J (2003): Five novel norcembranoids from Sinularia leptoclados. and S. parva.. Tetrahedron 59: 7337–7344. [CROSSREF]
- Alejandro MSM, Gustafson KR (2003): Marine pharmacology in 2000: Antitumor and cytotoxic compounds. Int J Cancer 105: 291–299.
- Anjaneyulu ASR, Raju KVS (1995): Secondary metabolites of a new soft coral of the genus Sinularia.of the Mandapam coast. Ind J Chem 34B: 463–465.
- Anjaneyulu ASR, Venkateswarlu R (1995): The chemical constituents of the soft coral-species of Sinularia. Genus: A review. J Sci Ind Res 54: 637–649. [CSA]
- Anjaneyulu ASR, Sagar KS (1996): Flexibiolide and dihydroflexibiolide, the first trihydroxycembranolide lactones from the soft coral Sinularia flexibilis. of the Indian Ocean. Nat Prod Lett 9: 12.
- Anjaneyulu ASR, Gowri PM (1999): A novel diketosesquiterpenoid from the soft coral Sinularia intacta. of the Indian Ocean. Ind J Chem 38B: 4–7.
- Anjaneyulu ASR, Sarada P (1999): Bishomoisomandapamate, a new tetracyclic diterpenoid from a new species of the Sinularia. genus of the Indian Ocean. J Chem Res 10 (S): 600–601.
- Anjaneyulu ASR, Rao GV, Sagar KS (1995a): Sandensolide, a new dihydroxycembranolide from the soft coral Sinularia sandensis. Verseveldt of the Indian Ocean. Nat Prod Lett 7: 183–190.
- Anjaneyulu ASR, Sagar KS, Venugopal JRV (1995b): Terpenoid and steroid constituents of the Indian Ocean soft coral Sinularia maxima.. Tetrahedron 51: 10997–11010.
- Anjaneyulu ASR, Rao GV, Rao NSK (1996): Sesqui and diterpenoids of the soft coral Sinularia hirta. of the Andaman and Nicobar Island. Ind J Chem 35(B): 815–818.
- Anjaneyulu ASR, Sagar KS, Rao GV (1997a): New cembranoid lactones from the Indian Ocean soft coral Sinularia flexibilis.. J Nat Prod 60: 9–12. [CSA], [CROSSREF]
- Anjaneyulu ASR, Krishnamurthy MR, Rao GV (1997b): Rare aromadendrane diterpenoids from a new soft coral species of Sinularia. genus of the Indian Ocean. Tetrahedron 53: 9301–9312. [CROSSREF]
- Anjaneyulu ASR, Venugopal JRV, Sarada P, Clardy J, Lobkovsky E (1998): Havellockate, a novel seco and spiro lactone diterpenoid from the Indian Ocean soft coral Sinularia granosa.. Tetrahedron Lett 39: 129–142.
- Anjaneyulu ASR, Gowri PM, Murthy MVRK (1999): New sesquiterpenoids from the soft coral Sinularia intacta. of the Indian Ocean. J Nat Prod 62: 1600–1604. [CROSSREF]
- Anjaneyulu ASR, Venugopal JRV, Sarada P (2000): On the novel cembranoids of the soft coral Sinularia granosa. of the Indian Ocean and their biogenesis. Ind J Chem 39B: 530–535.
- Antczak K, Kingston JF, Fallis AG, Hanson AW (1987): A general intramolecular Diels-Alder approach to tricyclic sesquiterpenes: Stereoselective total syntheses of (±)-sinularene and (±)-5-epi-sinularene. Can J Chem 65: 114–123.
- Beechan CM, Djerassi C, Finer JS, Clardy J (1978a): Terpenoids LXXIII. Sinularene, a sesquiterpene hydrocarbon based on a novel skeleton from the soft coral Sinularia mayi.. Tetrahedron Lett 28: 2395–2398.
- Beechan CM, Djerassi C, Eggert H (1978b): The sesquiterpenoids from the soft coral Sinularia mayi.. Tetrahedron 34: 2503–2508. [CROSSREF]
- Bowden BF, Coll JC, Mitchell SJ, Mulder J, Stokie GJ (1978): Studies of Australian soft corals IX. A novel nor-diterpene from the soft coral Sinularia leptoclados.. Aust J Chem 31: 2049–2056.
- Bowden BF, Coll JC, De Silva ED, De Costa MSL, Djura PJ, Mahendran M, Tapiolas DM (1983): Studies of Australian soft corals XXXI. Novel furanosesquiterpenes from several Sinularian. soft corals. Aust J Chem 36: 371–376. [CSA]
- Bowden BF, Coll JC, Wright AD (1989): Studies of Australian soft corals XLIV. New diterpenes from Sinularia polydactyla.. Aust J Chem 42: 757–763.
- Braekman JC, Daloze D, Tursch B, Hull SE, Declercq JP, Germain G, Van Meerssche M (1980): Chemical studies of marine invertebrates XXXVIII. Δ9(15)-Africanene, a new sesquiterpene hydrocarbon from Sinularia polydactyla.. Experientia 36: 893–893.
- Buckle PJ, Baldo BA, Taylor KM (1980): The anti-inflammatory activity of marine natural products 6-n-tridecylsalicylic acid, flexibilide and dendalone 3-hydroxybutyrate. Agents and Actions 10: 361–367.
- Chai M, Wang S, Dai C, Duh C (2000): A cytotoxic lobane diterpene from the Formosan soft coral Sinularia inelegans.. J Nat Prod 63: 843–844. [CSA], [CROSSREF]
- Coll JC (1992): The chemistry and chemical ecology of octocorals. Chem Rev 92: 613–631. [CROSSREF]
- Coll JC, Mitchell SJ, Stokie GJ (1977): Studies of Australian soft corals V. A novel furano-sesquiterpene acid from the soft coral Sinularia Gonatodes.. Tetrahedron Lett 18: 1539–1542. [CROSSREF]
- Coll JC, Liyanage N, Stokie GJ, Altena IV, Nemorin JNE, Sternhell S, Kazlauskas R (1978): Studies of Australian soft corals III. A novel furanoquinol from Sinularia lochomodes.. Aust J Chem 31: 157–162.
- Collins PA, Wege D (1979): The total synthesis of sinularene, a sesquitepene hydrocarbon from the soft coral. S. mayi. Aust J Chem 32: 1819–1826.
- Duh C, Hou R, Wang S, Chang T (1993): Formosan soft corals III. Cytotoxic norcembrene diterpenes from the soft coral Sinularia polydactyla.. Chin Pharm J 45: 399–407.
- Duh C, Hou R (1996): Cytotoxic cembranoids from the soft corals Sinularia gibberosa. and Sarcophyton trocheliophorum.. J Nat Prod 59: 595–598. [CSA], [CROSSREF]
- Duh C, Wang S, Tseng H, Sheu J (1998a): A novel cytotoxic biscembranoid from the Formosan soft coral Sinularia flexibilis.. Tetrahedron Lett 39: 7121–7122. [CROSSREF]
- Duh C, Wang S, Tseng H, Sheu J, Chiang M (1998b): Novel cytotoxic cembranoids from the soft coral Sinularia flexibilis.. J Nat Prod 61: 844–847. [CSA], [CROSSREF]
- Duh C, Wang S, Chia M, Chiang M (1999): A novel cytotoxic norditerpenoid from the formosan soft coral Sinularia inelegans.. Tetrahedron Lett 40: 6033–6035. [CROSSREF]
- Elsayed KA, Hamann MT (1996): A new cembranoid dimer from the Red Sea soft coral Sinularia gardineri.. J Nat Prod 59: 687–689. [CSA], [CROSSREF]
- Faulkner DJ (2002): Marine natural products. Nat Prod Rep 19: 1–49. [CSA]
- Goud TV, Reddy NS, Krishnaiah P, Venkateswarlu Y (2002): Spathulenol: A rare sesquiterpenoid from the soft coral Sinularia kavarattiensis.. Biochem Syst Ecol 30: 493–495. [CSA]
- Grace KJS, Zavortink D, Jacobs RS (1994): Inactivation of bee venom phospholipase A2 by a sesquiterpene furanoic acid marine natural product. Biochem Pharmacol 47: 1427–1434. [CSA], [CROSSREF]
- Guerrero PP, Read RW, Batley M, Janairo GC (1995): The structure of a novel cembranoid diterpene from a Philipine collection of the soft coral Sinularia flexibilis.. J Nat Prod 58: 1185–1191. [CSA], [CROSSREF]
- Haefner P (2003): Drugs from the deep: Marine natural products as drug candidates. Drug Discov Today 8: 536–544. [CSA], [CROSSREF]
- Hamada T, Kusumi T, Ishitsuka MO, Kakisawa H (1992): Structures and absolute configuration of new lobane diterpenoids from the Okinawan soft coral Sinularia flexibilis.. Chem Lett 3: 3–36.
- Hou R, Duh C, Chiang MY, Lin C (1995): Sinugibberol, a new cytotoxic cembranoid diterpene from the soft coral Sinularia gibberosa.. J Nat Prod 58: 1126–1130. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hsieh P, Chang F, Mcphail AT, Lee K, Wu Y (2003): New cembranolide analogues from the Formosan soft coral Sinularia flexibilis. and their cytotoxicity. Nat Prod Res 17: 409–418. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Iguchi K, Nishimura K, Yamazaki K, Iwashima M, Yamada Y (1992): New cembranolide diterpenes with a dimethylamino group from the Okinawan soft coral Sinularia. sp. Chem Lett 127–130. [CSA]
- Iguchi K, Kajiyama K, Yamada Y (1995): Yonarolide: A new marine norditerpenoid possessing a novel tricyclic skeleton, from the Okinawan soft coral of the genus, Sinularia.. Tetrahedron Lett 36: 8807–8808.
- Iguchi K, Kajiyama, Miyaoka H, Yamada Y (1996): Sinulariodiolide, a novel marine norditerpenoid from Okinawan soft coral of the genus, Sinularia. J Org Chem 61: 5998–6000. [CSA], [CROSSREF]
- Kamel HN, Fronczek FR, Fischer NH, Slattery M (2004): A novel metabolite from the hybrid soft coral Sinularia maxima. x S. polydactyla.: A biosynthetically mixed skeleton linking a cembrane and africanane terpenoid. Tetrahedron Lett. 45: 1995–1997. [CROSSREF]
- Karlsson R (1977): The structure and absolute configuration of sinulariolide, a cembranolide diterpene. Acta Cryst B33: 2027–2031.
- Kashman Y, Bodner M, Loya Y, Benayahu Y (1977): Cembranolides from marine origin (Red Sea), survey, and isolation of new sinulariolide derivatives. Isr J Chem 16: 1–3.
- Kashman Y, Bodner M, Finer-Moore JS, Clardy J (1980): Δ9(15)-Africanene, a new sesquiterpene hydrocarbon from the soft coral Sinularia erecta.. Experientia 36: 891–892.
- Kazlauskao R, Murphy PI, Wells RJ, Schonholzer P, Coll JC (1978): Cembranoid constituents from an Australian collection of the soft coral Sinularia flexibilis.. Aust J Chem 31: 1817–1824.
- Kelecom A (1999): Chemistry of marine natural products: Yesterday, today and tomorrow. An Acad Bras Cienc 71: 249–263.
- Kobayashi M (1988): Marine terpenes and terpenoids IV. Isolation of new cembranoid and secocembranoid lactones from the soft coral Sinularia mayi.. Chem Pharm Bull 36: 488–494. [CSA]
- Kobayashi M, Hamaguchi T (1988): Marine terpenes and terpenoids VI. Isolation of several plausible precursors of marine cembranolides from the soft coral, Sinularia mayi.. Chem Pharm Bull 36: 3780–3786. [CSA]
- Kobayashi M, Ishizaka T (1992): Marine terpenes and terpenoids. Part 15. Dimethylamine adducts of α-methylene-γ-lactonic marine cembranoids. J Chem Res(S.): 340–341.
- Kobayashi M, Ishizaka T, Miura N, Mitsuhashi H (1987): Marine terpenes and terpenoids III. Isolation and structures of two cembrane diols form the soft coral Sinularia mayi.. Chem Pharm Bull 35: 2314–2318. [CSA]
- Kobayashi M, Rao KMC, Krishna MN, Anjaneyulu V (1995): Marine terpenes and terpenoids. Part 19. Structure of a tetracyclic norcembranolide derivative isolated from the soft coral Sinularia dissecta.. J Chem Res (S.): 188–189.
- Kobbe WCM, Epstein S, Look SA, Rau GH, Fenical W, Djerassi C (1984): On the origin of terpenes in symbiotic associations between marine invertebrates and algae (Zooxanthellae). J Biol Chem 259: 8168–8173.
- Kusumi T, Uchida H, Ishitsuka MO, Yamamoto H, Kakisawa H (1988): Alcyonin, a new cladiellane diterpene from the soft coral Sinularia flexibilis.. Chem Lett 1077–1078.
- Lakshmi V, Schmitz FJ (1986): Metabolites from two soft corals from Guam: Sinularia leptoclados and Sinularia gyrosa.. J Nat Prod 49: 728–730. [CROSSREF]
- Lan J, Li J, Liu Z, Li Y, Chanb ASC (1999): The first total synthesis of (−) sinulariol-B and three other cembranoids. Tetrahedron Assym 10: 1877–1885. [CROSSREF]
- Lin C, Su J, Zeng L (2002): Crystal structure of a new cembranolide diterpene. Chem Res Chinese U18: 189–191.
- Missakian MG, Burreson BJ, Scheuer PJ (1975): Pukalide, a furanocembranolide from the soft coral Sinularia abrupta.. Tetrahedron 31: 2513–2515. [CROSSREF]
- Mori K, Suzuki S, Iguchi K, Yamada Y (1983): 8,11-Epoxy bridged cembranolide diterpene from the soft coral Sinularia flexibilis.. Chem Lett 1515–1516. [CSA]
- Nagaoka H, Iwashima M, Abe H, Iguchik K, Yama da Y (1992): Total synthesis of ( + )-mayolide A. Absolute structure of mayolide A, a secocembrane diterpenoids from the soft coral Sinularia mayi.. Chem Pharm Bull 40: 1742–1749. [CSA]
- Oppolzer W, Strauss HF, Simmons DP (1982): Stereoselective total synthesis of (±) sinularene and of (±) 5-episinularene via intramolecular type-I-magnesium-ene reaction. Tetrahedron Lett 23: 4673–4676. [CROSSREF]
- Oppolzer W, Begley T, Ashcroft A (1984): Application of the intamolecular magnesium-ene reaction to the stereocontrolled total syntheses of (±)-12-acetoxysinularene and (±)-5-epi-12-acetoxysinularene. Tetrahedron Lett 25: 825–828. [CROSSREF]
- Peter W, Michael SJ (2002): Synthesis of the C (1)-C (18) segment of lophotoxin and pukalide. control of 2-alken-ylfuran (E/Z.)-configuration. Org Lett 10: 1787–1790.
- Proksch P, Edrada RA, Ebel R (2002): Drugs from the sea- Current status and microbiological implications. Appl Microbiol Biotech 59: 125–134. [CSA], [CROSSREF]
- Radhika P, Rao PVS, Anjaneyulu V, Asolkar RN, Laatsch H (2002): Horiolide, a novel norditerpenoid from Indian Ocean soft coral of the genus Sinularia.. J Nat Prod 65: 737–739. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ramesh P, Venkateswarlu Y (2000): Unusual terpenoid constituents of the soft coral Sinularia dissecta.. J Chem Res (S): 48–50. [CSA]
- Ramesh P, Reddy NS, Venkateswarlu Y (1998): Rameswaralide, a novel diterpenoid from the soft coral Sinularia dissecta.. Tetrahedron Lett 38: 8217–8220. [CROSSREF]
- Ramesh P, Reddy NS, Rao TP, Venkateswarlu Y (1999): New oxygenated africanenes from the soft coral Sinularia dissecta.. J Nat Prod 62: 1019–1021. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ramesh P, Ravikanth V, Venkateswarlu Y (2001): New sesquiterpenoid from the soft coral Sinularia dissecta.. Ind J Chem 40B: 867–868.
- Rashid MA, Gustafson KR, Boyd MR (2000): HIV-Inhibitory cembrane derivatives from a Philippines collection of the soft coral Lobophytum. species. J Nat Prod 63: 531–533. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Reddy BSG, Rao VD, Rao BCh, Dhananjaya N, Kuttan R, Babu TD (1999): Isolation and structural determination of new sphingolipids and pharamcological activity of africanene and other metabolites from Sinularia leptoclados.. Chem Pharm Bull 47: 1214–1220. [PUBMED], [INFOTRIEVE], [CSA]
- Reddy NS, Goud TV, Venkateswarlu Y (2000): Acid catalysed rearrengment of Δ9(15)-africanene: A cytotoxic sesquiterpene. J Chem Res (M): 438–439. [CSA]
- Reddy NS, Goud TV, Venkateswarlu Y (2002): seco.-Sethukarailin, a novel diterpenoid from the soft coral Sinularia dissecta.. J Nat Prod 65: 1059–1060. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Rudi A, Dayan TL, Aknin M, Gaydou EM, Kashman Y (1998): Several new isoprenoids from the soft coral Sinularia erecta.. J Nat Prod 61: 872–875. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sanduja R, Sanduja SK, Weinheimer AJ, Alam H (1986): Isolation of the cembranolide diterpenes dihydroSinularin and 11-epi-sinulariolide from the marine mollusk Planaxis sulcatus.. J Nat Prod 49: 718–719. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Sato A, Fenical W, Qi-tai Z, Clardy J (1985): Norcembrene diterpenoids from Pacific soft corals of the genus Sinularia.. Tetrahedron 41: 4303–4308. [CROSSREF]
- Scheuer PJ (1978–1983): Marine Natural Products: Chemical and Biological Perspectives, Vols I–V. New York, Academic Press.
- Sheu J, Ahmed F, Shiue R, Dai C, Kuo Y (2002): Scabrolides A-D, four new norditerpenoids isolated from the soft coral Sinularia scabra.. J Nat Prod 65: 1904–1908. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Shoji N, Umeyama A, Arihara S (1993): A novel diterpenoid from the Okinawan soft coral Sinularia. sp. J Nat Prod 56: 1651–1653. [CSA], [CROSSREF]
- Su J, Yang JSR, Kuang Y, Zeng L (2000): A new cembranoids from the soft coral Sinularia capillpsa.. J Nat Prod 63: 1543–1545. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sui-jian Q, Qi C, Wen-lie P, An-long XJ (2002): Cytotoxic properties of cembranolides diterpenoids from soft coral Sinularia tenella. Li. Trop Oceanogr 21: 87–91. [CSA]
- Takaki H, Koganemaru R, Iwakawa Y, Higuchi R, Miyamato, T (2003): Inhibitory effect of norditerpenoids on LPS-induced TNF-α production from the Okinawan soft coral, Sinularia. sp. Biol Pharm Bull 26: 380–382. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Turner KE, Bowden BF, Stokie GJ, Howard CJ (1979): (4R.*, 8S.*, 11R.*, 13S.*, 14R.*)-8,11-Epoxy-14-hydroxy-11-methyl-4-(1-methylvinyl)-6,9-dioxocyclotetradec-1-ene-1,13-caebolactone. Acta Cryst B35: 1283–1284.
- Tursch B (1976): Some recent development in the chemistry of Alcyonaceans. Pure Appl Chem 48: 1–6.
- Tursch B, Braekman JC, Daloze D, Fritz P, Kelecom A, Karlsson R, Losman D (1974): Chemical studies of marine invertebrates VIII. Africanol, an unusual sesquiterpene from Lemnalia Africana.. Tetrahedron Lett 9: 747–750. [CSA], [CROSSREF]
- Tursch B, Braekman JC, Daloze D, Herin M, Karlsson R, Losman D (1975): Chemical studies of the marine invertebrates XI. Sinulariolide, a new cembranolide diterpene from the soft coral Sinularia flexibilis.. Tetrahedron 31: 129–133. [CROSSREF]
- Tursch B, Braekman JC, Daloze D, Kaisin M (1978): Terpenoids from coelenterates. In: Scheuer, PJ, ed. Marine Natural Products: Chemical and Biological Perspectives. New York, Academic Press, pp. 247–296.
- Uchio Y, Nabeya H, Nakayama M, Hayashi S, Hase T (1981): Cembrene and mayol, two new cembranoid diterpene from the soft coral Sinularia mayi.. Tetrahedron Lett 22: 1689–1690. [CROSSREF]
- Venkateswarlu Y, Biabani MAF, Reddy MVR, Rao TP, Kunwar AC, Faulkner DJ (1994): Madapamate, a diterpenoid from the soft coral Sinularia dissecta.. Tetrahedron Lett 35: 2249–2252. [CROSSREF]
- Venkateswarlu Y, Ramesh P, Reddy PS, Jamil K (1999a): Microbial transformation of Δ9(15)-africanene. Phytochemistry 52: 1275–1277. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Venkateswarlu Y, Sridevi KV, Rao MR (1999b): New furanocembranoid diterpenes from the soft coral Sinularia maxima.. J Nat Prod 62: 756–768. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Venkateswarlu V, Reddy SN, Venkatesham U (2001): Novel bioactive compounds from the soft corals: chemistry and biomedical applications. In: Fingerman M, Nagabhushanam R, eds. Recent Advances in Marine Biotechnology, Vol. 6: Bio-organic Compounds and Biomedical Applications. Science Publisher, Inc., NH, pp. 101–129.
- Verseveldt J (1980): A Revision of the Genus Sinularia (Octocorallia, Alcyonacea.). Brill Leiden:, Leiden, The Netherlands, pp. 1–128.
- Wahlberg I, Eklund A (1992): Cembranoids, pseudopteranoids, and cubitanoids of natural occurrence. Prog Chem Org Nat Prod 59: 141–294. [CSA]
- Weinheimer A, Matson JA, Hossain MB, Vander Helm D (1977): Marine anticancer agents: Sinularin and dihydrosinularin, new cembranolides from the soft coral, Sinularia flexibilis.. Tetrahedron Lett 34: 2923–2926. [CROSSREF]
- Weinheimer AJ, Spraggins RL (1969): Chemistry of coelenterates XV. Occurrence of two new prostaglandin derivatives (15-epi-PGA2 and its acetate, methyl ester) in the gorgonian Plexaura homomalla.. Tetrahedron Lett 15: 5185–5188. [CROSSREF]
- Williams DH, Faulkner DJ (1996): The practical syntheses of an anti-inflammatory sesquiterpene furoic acid from Sinularia. sp. Tetrahedron 52: 4245–4256. [CROSSREF]
- Yamada K, Ujiie T, Yoshida K, Miyamoto T, Higuchi R (1997): Sinulobatins A-D, new amphilectane diterpenoids from the Japanese soft coral Sinularia nanolobata.. Tetrahedron 53: 4569–4578. [CROSSREF]
- Yue X, Lan J, Li J, Liu Z, Lin Y (1999): An efficient total synthesis of (±)-sinulariol B. Tetrahedron 55: 133–140. [CROSSREF]