ABSTRACT
In this investigation, 185 plant samples representing more than 30 plant families collected from the Malaysian forests were assessed for their ability to inhibit specific radioligand binding to 5HT1a, GABAB, and dopamine (D2S) receptors. For this study, 96-well microplate filtration assays were adopted, and the screening parameters including screening window factor (z factor) and z′ factor indicated that the assays adopted were robust and suitable for medium-throughput screening (MTS). z factor also indicated that data on plant extracts at 10 µg/well were more reliable compared to those obtained from 100 µg/well. Therefore, only data at 10 µg/well in duplicate were used in the determination of actives. In the preliminary screen, 23 plant extracts were found to show activity (50% or higher level of inhibition over the mean of all samples for a given plate) in either one or both of the duplicates. Of these, seven were reconfirmed to be active on 5HT1a receptor in the hit confirmation. The active plant extracts were isolated from Popowia odoardoi. Diels (Annonaceae) (leaf and stem), Artabotrys roseus. Boerl. (Annonaceae) (bark), Litsea elliptibacea. Merr. (Lauraceae) (bark), Decaspermum fruticosum. Forst. (Myrtaceae) (bark), Dyera costulata. (Miq.) Hook. f. (Apocynaceae) (leaf), and Irvingia malayana. Oliv. (Simaroubaceae) (leaf). However, none of the plant extracts tested were active on either GABAB or D2S receptors.
Introduction
Disorders of the central nervous system (CNS) are the cause of a number of common diseases throughout the world, which include migraine, sleeping disorders, obsessive disorders, schizophrenia, Alzheimer disease, epilepsy, and Parkinson disease (Laurence et al., Citation1997; Lefkowitz et al., Citation1990). Some of these disorders are related to neurotransmitters such as acetylcholine, glutamic acid, dopamine, 5-hydroxytryptamine, γ.-aminobutyric acid, glycine, benzodiazepine, noradrenaline, and histamine, and their receptors (Lefkowitz et al., Citation1990).
Historically, plants have provided important CNS active compounds including morphine, codeine, reserpine, and caffeine (Cragg et al., Citation1997; Grabley & Thiericke, Citation1999; Evans & Evans, Citation2002). However, the majority of the plants have not been investigated to any great extent for their pharmacological activities, and it is believed that plants can provide new drug leads for the treatment of CNS diseases (Zhu et al., Citation1996). The recent developments in radioactive ligand-receptor binding assays offer a rapid turnover of the screening process and hence may expedite the process in the search of novel drug molecules or templates (Zhu et al., Citation1996; Marks et al., Citation2002). These developments include the miniturization and automation of the screening process (Oldenburg et al., Citation2001; Menke, Citation2002). In the current study, we evaluated CNS activities of some Malaysian plants using competitive radioligand receptor binding assays, and the receptor activities assessed were 5-hydroxy tryptamine (5HT), GABA, and dopamine. The aim was to qualitatively identify plants that exhibit significant CNS activities for further bioassay-guided isolation of active constituents.
Materials and Methods
Materials
[3H]-Spiperone, [3H]-CGP 54626, and [3H]-8-OH-DPAT were obtained from Amersham Pharmacia Biotech (Little Chalfont, Buckinghamshire, UK), Tocris (Ellisville, MO, USA), and Perkin Elmer (Boston, MA, USA), respectively. Haloperidol, GABA, and metergoline were purchased from RBI (Natick, MA, USA). Unless stated otherwise, all other reagents of analytical grade were obtained through standard commercial sources.
Deep-well titer plate polypropylene (Beckman Coulter, Fullerton, CA, USA), Multiscreen Harvest plates-GF/C (Millipore, Billerica, MA, USA), TopSeal-A (Packard, Meriden, CT, USA), Bottom Seal (Millipore), and MicroScint-O (Packard) were purchased. SignalScreen membranes from cells expressing dopamine (D2S: co-expressed in sf9 cells with Gα3β1γ2; no. 6110138), GABAB 1a (co-expressed in HEK 293 cells with GABAB 2; no. 6110557), and 5-HT1a (expressed in CHO cells; no. 6440501) human receptors were supplied by Biosignal (Montreal, Quebec, Canada).
Plant samples and crude extracts dilution
A total of 185 plant samples were collected from Forest Research Institute Malaysia, Kuala Lumpur, Malaysia (voucher no.: 5-digit series) and Tabun Wildlife Reserve, Sabah, Malaysia (voucher no.: 6-digit series). The voucher specimens were kept at the herbaria of Forest Research Institute Malaysia, Kuala Lumpur, Malaysia (5-digit series), and Forest Research Center, Sepilok, Sandakan, Sabah, Malaysia (6-digit series). The plant samples were dried, ground, and macerated (100 g) with sufficient methanol in conical flasks for 7 days with sonication (2 × 30 min). The methanolic solution was filtered, solvent removed in vacuo. and freeze-dried, giving a dried residue that was weighed and kept at − 20°C in sample vials until use.
DMSO (1.25 ml) was added to 5 mg of crude plant extracts and vortexed vigorously, giving an initial concentration of 4 mg/ml. The extracts were tested at 100 µg/assay point and 10 µg/assay point.
Receptor binding assays
The receptor binding assays were carried out according to the recommended protocols (Biosignal). Briefly, the membranes were thawed on ice and diluted to 1 SignalScreen Unit per 500 µl with the appropriate binding buffer (D2S: 50 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 1 mM EDTA, 120 mM NaCl; GABAB 1a + 2: 50 mM Tris-HCl, pH 7.4, 2.5 mM CaCl2; 5HT1a: 50 mM Tris-HCl, pH 7.4, 10 mM MgSO4, 0.5 mM EDTA, 0.1% ascorbic acid). The reference ligands and radioligands were diluted at 22 × the final concentration in 100% DMSO and in binding buffer, respectively ().
Table 1. Final concentrations of radioligands and reference ligands.
The diluted membranes (500 µl) were added to each well of the deep-well plate, followed by addition of 25 µl of DMSO (total value, 5 wells), reference compound (nonspecific value, 3 wells) or crude extracts to the corresponding well in the deep-well plate. The reaction was initiated by adding 25 µl of radioligand to each well. TopSeal-A was applied to the plate, vortexed gently, and incubated at 27°C while shaking for 60 min. During incubation, the Multiscreen Harvest plates were preincubated in 0.3% aqueous polyethyleneimine (D2S), 50 mM Tris pH 7.4 (GABAB 1a + 2), or 0.3% polyethyleneimine in binding buffer (5HT1a). The reaction mixture was then filtered over the presoaked Multiscreen Harvest plate using a Tomtec Harvester and washed 9-times with 500 µl of cold 50 mM Tris-HCl, pH 7.4, at 4°C and air-dried for 30 min at room temperature under a fume hood. A bottom seal was applied to the Multiscreen Harvest plate, 25 µl of MicroScint-O added to each well, and followed by sealing of the top using TopSeal-A. The plate was then counted for 60 s per well using TopCount NXT (Packard) with a count delay of 60 s.
Data analysis
Data analysis on saturation experiment using the non-linear least-squares regression method was performed using PRISM Software (GraphPad Software, Inc., San Diego, CA, USA) and the results are presented as mean ±SEM.z′ factor analysis, originally described by Zhang et al. (Citation1999) to evaluate the quality of the screening assays, was performed on replicate (> 150) test wells and NSB wells and the conditions were as described above.
To calculate the percentage of inhibition of specific binding to 5HT, GABA, or dopamine receptors in the presence of the test compounds, a standard data reduction algorithm was used as shown below:
where B. = binding in the presence of test extract, NSP. = nonspecific binding in the presence of excess inhibitor, and T. = total binding.
Results and Discussion
The respective binding of [3H]-8-OH-DPAT, [3H]-CGP 54626, and [3H]-spiperone to 5HT1a, GABAB 1a + 2, or D2S receptors were with high affinity and saturable (). The dissociation constants (K.d) were estimated to be 0.12 nM ([3H]-spiperone; D2S), 6.22 nM ([3H]-CGP 54626; GABAB), and 0.3 nM ([3H]-6-OH-DPAT; 5HT1a) (). K.i for the respective competitive/reference ligands were 13.6 nM (haloperidol), 22.8 nM (GABA), and 6.3 nM (metergoline). In the time-course experiments, association of radioligands were completed at about 30 min; this clearly suggests 60 min incubation time was adequate ().
Figure 1 (a) Saturation curve for D2S receptors. (b) Saturation curve for GABAB 1a+2 receptors. (c) Saturation curve for 5HTla receptors.
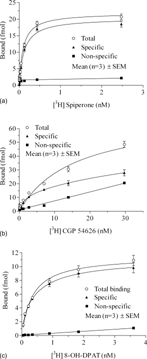
Figure 2 Time course of association of [3H]-spiperone to D2S receptors. (b) Time course of association of [3H]-CGP 54626 to GABAB 1a+2 receptor. (c) Time course of association of [3H]-6-OH-DPAT to 5HTla receptors.
![Figure 2 Time course of association of [3H]-spiperone to D2S receptors. (b) Time course of association of [3H]-CGP 54626 to GABAB 1a+2 receptor. (c) Time course of association of [3H]-6-OH-DPAT to 5HTla receptors.](/cms/asset/338537eb-3673-4c6f-9604-4e577362334a/iphb_a_54082_f0002_b.gif)
Table 2. K.d of radioligands and K.i of competitive ligands.
Interplate variations of total binding, specific binding, and nonspecific binding were analyzed from 5 points per microplate of a total of 12 microplates. For total binding and specific binding for each receptor binding assay, the %CV is less than 10, whereas the values were higher for nonspecific binding (). The results show interplate variation is minimal and acceptable for the purpose of high throughput screening (HTS) and is further supported by the z′ factor (see below).
Table 3. Interplate receptor binding variations.
The quality of the assays was tested by performing z′-factor analysis as described by Zhang et al. (Citation1999). Assays with a z′ factor between 0.5 and 1.0 are considered to be reliable, robust, and suitable for HTS. In each case, the z′ factor determined was more than 0.5 (), indicating the assays adopted are suitable for HTS purposes (Zhang et al., Citation1999; Oldenburg et al., Citation2001).
Table 4. S/N and z′ factor.
In the preliminary screening, the plant extracts were tested at 10 and 100 µg/assay point as, under these conditions, the samples remained soluble and proper filtration was achieved. The samples were screened in duplicate at both concentrations for the three receptors and the percent inhibition averaged. The calculated values of z factor at 10 µg/well were generally larger compared to those of 100 µg/well for the assay procedures; this indicates the screening results of 10 µg/well are more reliable. Furthermore, at 100 µg/well, most of the samples showed a high level of inhibition, thus making the determination of actives difficult. For these reasons, only the data at 10 µg/well were used in the determination of actives. Actives were determined by choosing any extract that showed 50% or greater inhibition over the mean of all samples for a given plate.
From the preliminary screens, 23 plant extracts were found to show activity (none on D2S, 9 on 5HT1a, and 14 on GABAB 1a + 2) in either one or both of the duplicates (). All the plant extracts that exhibited activity in only one of the duplicates were found to be false positives. Of those extracts that showed activity in the preliminary screen, seven were reconfirmed to be active (showed 50% or greater inhibition) on 5HT1a receptors in the hit confirmations. The active plants are Popowia odoardoi. Diels (Annonaceae), Artabotrys roseus. Boerl. (Annonaceae), Litsea elliptibacea. Merr. (Lauraceae), Decaspermum fruticosum. Forst. (Myrtaceae), Dyera costulata. (Miq.) Hook. f. (Apocynaceae), and Irvingia malayana. Oliv. (Simaroubaceae) (). However, none of the plant extracts tested show high receptor binding activity against GABAB and D2S receptors. The active plants have now been selected for further testing and bioassay-guided fractionation to identify active constituents.
Table 5. Preliminary screening on CNS receptor activities of plant extracts of some Malaysian plant species.
Table 6. Hit confirmation on 5HT1a receptor activity of plant extracts of some Malaysian plant species.
Acknowledgment
This project was supported by Biotechnology Directorate, Ministry of Science, Technology and the Environment, Malaysia (IRPA 26-02-06-0127).
References
- Cragg GM, Newman DJ, Snader KM (1997): Natural products in drug discovery and development. J Nat Prod 60: 52–60. [PUBMED], [INFOTRIEVE], [CSA]
- Evans WC, Evans D (2002): Trease & Evan's Pharmacognosy, 15th ed. London, WB Saunders.
- Grabley S, Thiericke R (1999): The impact of natural products on drug discovery. In: Grabley S, Thiericke R, eds. Drug Discovery from Nature. Berlin, Springer-Verlag, pp. 3–37.
- Laurence DR, Bennett PN, Brown MJ (1997): Clinical Pharmacology, 8th ed. London, Churchill Livingstone, pp. 285–372.
- Lefkowitz RJ, Hoffman BB, Taylor P (1990): Neurohumoral transmission: The autonomic and somatic motor nervous systems. In: Gilman AG, Rall TW, Nites AS, Taylor P, eds. The Pharmacological Basis of Therapeutics. New York, Pergamon Press, pp. 84–121.
- Marks JS, Burdette DS, Giegel DA (2002): Homogeneous techniques for monitoring receptor-ligand interactions. In: Janzen WP, ed. High Throughput Screening. Methods and Protocols. Totowa, NJ, Humana Press, pp. 51–63.
- Menke KC (2002): Unit automation in high throughput screening. In: Janzen WP, ed. High Throughput Screening. Methods and Protocols. Totowa, NJ, Humana Press, pp. 195–212.
- Oldenburg KR, Kariv I, Zhang JH, Chung TDY, Lin S (2001): Assay miniaturization. In: Seethala R, Fernandes PB, eds. Handbook of Drug Screening. New York, Marcell Dekker Inc., pp. 525–562.
- Zhang JH, Chung TD, Oldenburg KR (1999): A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4: 67–73. [PUBMED], [INFOTRIEVE]
- Zhu M, Bowery NG, Greengrass PM, Phillipson JD (1996): Application of radioligand receptor binding assays in the search for CNS active principles from Chinese medicinal plants. J Ethnopharmacol 54: 153–164. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
