Abstract
Five Bulgarian Amaryllidaceae species; Pancratium maritimum. L., Sternbergia colchiciflora. W. K., Galanthus nivalis. L., Galanthus elwesii. Hook., and Leucojum aestivum. L., were analyzed for their high-performance liquid chromatography (HPLC) profiles of phenolic acids. An improved HPLC method was developed for the simultaneous determination of seven phenolic acids. Protocatechuic, 4-hydroxybenzoic, vanillic, caffeic, syringic, p.-coumaric, and ferulic acids were identified in the studied species. Protocatechuic, 4-hydroxybenzoic, vanillic, and syringic acids are reported for the first time in Amaryllidaceae. Also, this is the first report on phenolic acid composition in the genus Pancratium., as well as in Galanthus elwesii, Sternbergia colchiciflora., and Pancratium maritimum..
Keywords:
Introduction
The family Amaryllidaceae has attracted considerable attention due to synthesis of pharmaceutically important alkaloids (Lewis, Citation2001; Sidjimova et al., Citation2003; Zhong, Citation2003; Berkov et al., Citation2004). In contrast to alkaloids, there are scanty data on phenolic compounds in this family. Recently, data on flavonoid constituents of Pancratium maritimum. L. and Crinum bulbisperumum. Milne have been reported (Youssef et al., Citation1998; Ramadan et al., Citation2000). Only a few reports have been published concerning the presence of phenolic acids in Amaryllidaceae, using thin-layer chromatography (TLC) (Bate-Smith, Citation1968). High-performance liquid chromatography (HPLC) is commonly used for the determination of phenolic acids in different matrices (Lo & Chung, Citation1999; Ogiwara et al., Citation2002). To the best of our knowledge, no HPLC method has been reported for separation of phenolic acids in Amaryllidaceae species. It is well-known that many phenolic acids (caffeic, chlorogenic, ferulic, gallic, ellagic) exert potential health-promoting effects as antioxidants and antimutagenic and anticarcinogenic agents (Ibern-Gómez et al., Citation2002; Maciejewicz et al., Citation2002).
In this study, we report the separation and identification of phenolic acids in ethanol extracts from Sternbergia colchiciflora. W. K., Galanthus nivalis. L., Galantus elwesii. A. K., Leucojum aestivum. L., and Pancratium maritimum. L. by HPLC.
Materials and Methods
Plant material
The plant materials (aerial and underground parts) were collected from the Black Sea region, Bulgaria, in March–April 2003. Aerial parts of P. maritimum. were not available at the time of collecting. Vouchers of the plant samples used for this study were deposited in the Herbarium of the Institute of Botany, Bulgarian Academy of Sciences.
Sample preparation
The plant material was extracted with 90% EtOH (5:1) in triplicate. The combined etanol extracts were evaporated in vacuo.. The concentrated viscous extracts were diluted with 2% H2SO4 and extracted 3 × with CHCl3. Chloroform was evaporated, and the residue was subjected to HPLC analysis.
Chromatographic equipment and conditions
The chromatographic analyses were performed on a Varian (Walnut Creek, CA, USA) chromatographic system, which includes a tertiary pump Model 9012, Rheodyne injector with 20-µl sample loop, UV-VS detector Model 9050 set at 280 and 310 nm according to the UV absorption maximum of the compounds determined, Varian Star Chromatography workstation, and computer software (version 4.5) for controlling the system and collecting the data. A reversed-phase Hypersil ODS RP18, 5 µm, 250 × 4.6 mm I.D. column, (Chandon, Runcom, UK) column equipped with a precolumn 30 × 4.6 mm (Interchim, France) filled with the same stationary phase was used and maintained at 27°C.
The mobile phase was comprised of solvent (A): 25 mM potassium dihydrogen phosphate adjusted to pH 2.98 by phosphoric acid; and solvent (B) : methanol. The studied compounds were separated within 40 min by linear profile starting from 3% (B) to 40% (B) at a flow rate of 1.3 ml/min.
Identification of phenolic acids
Identification of each compound was performed by its retention time and by spiking with standards under the same conditions. Additionally, each sample was monitored at two different wavelengths, 280 and 310 nm selected on the specific UV absorption of the assayed compounds, and the chromatograms were compared. Detection at 310 nm corresponds mainly to caffeic, p.-coumaric, and ferulic acids.
Standards and chemicals
The standards of the phenolic acids were obtained from Extrasynthese (Genay, France) and Fluka (Buchs, Switzerland). The organic solvents HPLC-grade and analytical-grade potassium dihydrogen phosphate and o.-phosphoric acid were provided by Merck (Darmstadf, Germany).
Results and Discussion
HPLC separation of phenolic acids was tested on different types of reversed-phase columns. Best results were obtained on a Hypersil ODS RP18 column where seven phenolic acids were resolved applying a gradient elution mode. Protocatechuic (tR = 13.81), 4-hydroxybenzoic (tR = 20.56), vanillic (tR = 26.42), caffeic (tR = 28.00), syringic (tR = 30.43), p.-coumaric (tR = 36.52), and ferulic (tR = 40.02) acids were separated and identified in the extracts of studied species (). The relative standard deviations (RSDs) of the repeatability and the reproducibility were ≤ 1.82% and ≤ 3.94%, respectively.
Figure 1 HPLC chromatogram of the phenolic acids from Sternbergia colchiciflora. (aerial part). 1, protocatechuic acid; 2, 4-hydroxybenzoic acid; 3, vanillic acid; 4, caffeic acid; 5, syringic acid; 6, p.-coumaric acid; 7, ferulic acid.
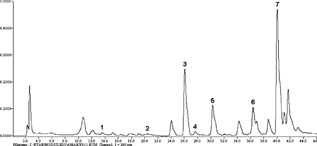
Five Amaryllidaceae species were examined for their HPLC profiles of phenolic acids. There were no considerable differences in the phenolic acids composition in the aerial parts of Sternbergia colchiciflora., Galanthus nivalis., Galanthus elwesii., and Leucojum aestivum.. The ferulic and vanillic acids dominated the phenolic acid profiles of all the studied samples, while protocatechuic acid was found in trace amounts. The ferulic acid reached 74% of the phenolic acid mixture in G. nivalis.. Syringic and p.-coumaric acids were also detected in the samples ().
Table 1.. Phenolic acid distribution in Amaryllidaceae species
In contrast to aerial parts, the underground parts of Sternbergia colchiciflora., Galanthus elwesii., and Pancratium maritimum. have qualitative and quantitative differences in their phenolic acids profiles. p.-Coumaric and ferulic acids were not detected in the bulbus of G. elwesii.. Caffeic acid was absent in the underground part of P. maritimum., whereas syringic acid is present with considerably higher percentage in this plant as compared to the other species (76% of the phenolic acids mixture).
The phenolic acid profiles in the aerial and underground parts of Sternbergia colchiciflora. are identical. In contrast, the aerial and underground parts of Galanthus elwesii. have different quantitative and qualitative profiles of the phenolic acids. Ferulic and p.-coumaric acids were not detected in the underground part, while in the aerial part, these phenolic acids were found.
In conclusion, the phenolic acids of the plant extracts can be successfully separated and identified by reversed-phase HPLC (RP-HPLC) with a gradient elution mode. The method proposed allows a simultaneous determination of the naturally occurring organic acids in a variety of plant species, providing an acceptable limit of detection and repeatability. The results obtained revealed the presence of protocatechuic, 4-hydroxybenzoic, vanillic, caffeic, syringic, p.-coumaric, and ferulic acids. Protocatechuic, 4-hydroxybenzoic, vanillic, and syringic acids are reported for first time in Amaryllidaceae. This is a first report on phenolic acid constituents of the genus Pancratium. as well as for Galanthus elwesii., Sternbergia colchiciflora., and Pancratium maritimum..
References
- Bate-Smith EC (1968): The phenolic constituents of plants and their taxonomic significance. J Linn Society ( Bot.) 60: 325–383.
- Berkov S, Sidjimova B, Popov S, Evstatieva L (2004): Intraspecies variability in alkaloid metabolism in Galanthus elwesii.. Phytochemistry 65: 579–586. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Ibern-Gómez M, Andrés-Lacueva C, Lamuela-Raventós RM, Waterhouse AL (2002): Rapid HPLC method for phenolic compounds in red wines. Am J Enol Vitic 53: 218–221.
- Lewis JR (2001): Amaryllidaceae, sceletium, imidazole, oxazole, thiazole, peptide and miscellaneous alkaloids. Nat Prod Rep 18: 95–128. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lo HH, Chung JG (1999): The effects of plant phenolics, caffeic acid, chlorogenic acid and ferulic acid on arylamine N.-acetyltransferase activities in human gastrointestinal microflora. Anticancer Res 19: 133–139. [PUBMED], [INFOTRIEVE], [CSA]
- Maciejewicz W, Daniewski M, Dzido T, Bal K (2002): GC-MS and HPLC analysis of phenolic acids extracted from propolis and from Populus nigra. bud exudates. Chem Anal 47: 21–30.
- Ogiwara T, Satoh K, Kadoma Y, Murakami Y, Unten S, Atsumi T, Sakagami H, Fujisawa S (2002): Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res 22: 2711–2717. [PUBMED], [INFOTRIEVE], [CSA]
- Ramadan M, Kamel M, Ohtani K, Kasai R, Yamasaki K (2000): Minor flavonoids from Crimun bulbispertum. bulbs. Phytochemistry 54: 891–896. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sidjimova B, Berkov S, Popov S, Evstatieva L (2003): Galanthamine distribution in Bulgarian Galanthus. species. Pharmazie 58: 936–937.
- Youssef D, Ramadan M, Khalifa A (1998): Acetophenones, a chalcone, a chromone and flavonoids from Pancratium maritimum.. Phytochemistry 49: 2579–2583. [CROSSREF]
- Zhong J (2003): Amaryllidaceae and sceletium alkaloids. Nat Prod Rep 20: 606–614. [CSA], [CROSSREF]
