Abstract
The chemoprotective effect of Phyllanthus maderaspatensis. Linn (PME) was studied on cisplatin-induced nephro- and genotoxicity in male Swiss albino mice. The treatment of mice with different doses of PME (400 and 600 mg/kg body weight) for 7 days before the administration of a single i.p. dose of cisplatin (5 mg/kg) exhibited significant chemoprotective activity. Renal dysfunction was evaluated biochemically by measuring the concentration of blood urea nitrogen (BUN) and serum creatinine and histologically by light microscopy. Genotoxicity was evaluated by the bone marrow micronucleus assay. A single dose of cisplatin significantly elevated the levels of blood urea nitrogen, serum creatinine, and the kidney to body weight ratio, but pretreatment with PME (600 mg kg−1 day−1) for 7 days significantly attenuated the cisplatin-induced nephrotoxicity. The frequency of micronucleated polychromatic erythrocytes (MNPCEs) in the bone marrow was determined at 24 h after the administration of cisplatin. After administration of cisplatin, the frequency of MNPCEs distinctly increased. In mice treated with PME before cisplatin application, there was a decrease in the number of MNPCEs when compared with mice injected with only cisplatin. Ethanol extract of PME thus has a marked free radical scavenging effect indicating its antioxidative property. The results suggest that the ethanol extract of PME has a protective effect against cisplatin-induced nephro- and genotoxicity through its antioxidant property.
Introduction
The use of chemotherapy in the treatment of cancer has opened new possibilities for improvement of the quality of life of cancer patients. Despite its success, treatment with some of the most effective anticancer drugs shows a number of symptoms of direct toxicity. Additionally, many anticancer drugs have been shown to be mutagenic, teratogenic, and carcinogenic in experimental systems. Second malignancies are also known to be associated with several therapeutic treatments (Sorsa et al., Citation1985; IARC, Citation1987).
Cisplatin (cis.-dichlorodiammineplatinum-II) is a potent chemotherapeutic agent that has gained widespread use against various malignant tumors in different experimental animals (Kociba et al., Citation1970) and in a variety of human malignancies (Prasad & Giri, Citation1994). Many of the biological properties and effects of cisplatin have been well documented (Loehrer & Einhorm, Citation1984; Rosenberg, Citation1985) with numerous reports indicating that the cellular DNA could be the primary target in its anticancer activity (Pinto & Lippard, Citation1985; Chu, Citation1994). High doses of cisplatin are more effective for the suppression of cancer (Kociba et al., Citation1970; Prasad & Giri, Citation1994). This high-dose chemotherapy, however, produces untoward side effects of nephrotoxicity, bone marrow toxicity, gastrointestinal toxicity, ototoxicity, and peripheral neuropathy (Loehrer & Einhorm, Citation1984; Pinto & Lippard, Citation1985; Rosenberg, Citation1985; Prasad & Giri, Citation1994). It has been suggested that oxygen free radicals play an important role in cisplatin toxicity (Zamble & Lippard, Citation1995). Cisplatin chemotherapy induces a decrease in plasma antioxidant levels, leading to a failure of the antioxidant defense against free radical damage generated by antitumor drugs (Masuda et al., Citation1994). One of the approaches to deal with this problem is, therefore, to search for suitable antimutagens. During the past few years, considerable attention has been paid to investigations of naturally occurring agents that are able to stimulate defense mechanisms (Surh, Citation1999).
Plants have limitless ability to synthesize aromatic substances such as polyphenols, mainly flavonoids and phenolic acids, which exhibit antioxidant properties due their hydrogen-donating and metal-chelating capacities. It is known that many plant infusions have a large number of these molecules and, hence, it is reasonable to investigate whether plants have the capacity to prevent the genotoxic potency of specific mutagens or carcinogens from different categories that are known to generate free radicals in nontumor cells both in vivo. and in vitro..
Plants of the genus Phyllanthus. have been widely used in traditional medicine in China, the Philippines, Nigeria, East and West Africa, the Caribbean, and Central and Latin America for the treatment of different types of diseases (Thyagarajan et al., Citation1988; Unander et al., Citation1990Citation1995; Weijl et al., Citation1997). Phyllanthus maderaspatensis. Linn (Euphorbiaceae) is a traditional herbaceous medicinal plant. The leaves are expectorant and diaphoretic. The seeds have a bad taste and are carminative, laxative, tonic to the liver, diuretic, and useful in bronchitis, ear ache, griping, ophthalmia, and ascites. In South India, an infusion of the leaves is given for headache (Kirthikar & Basu, Citation1999). The plant has been shown to be effective in protecting acetaminophen-induced liver damage by elevating the antioxidant defense system (Asha & Pushpangadan, Citation1998; Asha et al., Citation2004).
The current study was, therefore, undertaken to assess the effect of Phyllanthus maderaspatensis. against cisplatin-induced geno- and nephrotoxicity and in vitro. antioxidant activity.
Materials and Methods
Plant material
Botanically identified (NISCOM, New Delhi, India, ref. no. RHM/F-3/98/consult. 178) whole plant material was supplied by Natural Remedies Pvt Limited (Bangalore, India). A voucher specimen (no. TIFAC 04) has been deposited at the herbarium of the J.S.S College of Pharmacy (Ootacamund, India).
Drugs and chemicals
Cisplatin was purchased from Dabur Pharmaceuticals (New Delhi, India). α.,α.-Diphenyl-β.-picryl-hydrazyl (DPPH), fetal calf serum (FCS), and acridine orange were purchased from Sigma Chemical Co. (St. Louis, MO, USA). N.,N.-Dimethyl p.-phenylendiamine dihydrochloride (DMPD) was obtained from Fluka (Switzerland). Diagnostic kits were obtained from Merck (Mumbai, India) Pvt. Ltd (Mumbai, India). All other chemicals used in the experiment were of analytical grade.
Preparation of the ethanol extracts
The air-dried and finely ground plant material was extracted by using the method described elsewhere (Sokmen et al., Citation1999). The sample, weighing about 100 g, was extracted in a Soxhlet apparatus with 70% ethanol at 60°C for 6 h. The extract was filtered and concentrated in vacuo. at 45°C (yield 5.6 g). It was then lyophilized and kept in the dark at 4°C until tested. Henceforth, the extract of P. maderaspatensis. will be called PME.
Animals
Male Swiss albino mice 10–12 weeks old, weighing 25–30 g, were obtained from National Institute of Nutrition (NIN; Hyderabad, India). Institutional ethical laws on animal use and care were complied with in all experiments. Animals were housed in plastic cages at 22 ± 1°C, 60 ± 10% humidity, and 12/12 h light/dark cycle during 2 weeks of acclimatization to laboratory conditions and through the entire experimental period. Water was available ad libitum., and the animals were fed with conventional laboratory diet (Hindustan Lever Limited, Mumbai, India).
Experimental protocol
The animals were divided into six groups (Groups 1–6) of six animals each:
Group 1: normal control, which received only the standard diet
Group 2: treated with PME (600 mg kg−1 day−1, p.o., for 7 days)
Group 3: positive control, which received a single dose of cisplatin (5 mg/kg, i.p.)
Group 4: treated with a single i.p. dose of cisplain 2 h after the last dose of PME (200 mg kg−1 day−1, p.o., for 7 days)
Group 5: treated with a single i.p. dose of cisplatin, 2 h after the last dose of PME (400 mg kg−1 day−1, p.o., for 7 days)
Group 6: treated with a single i.p. dose of cisplatin, 2 h after the last dose of PME (600 mg kg−1 day−1, p.o., for 7 days)
Twenty-four hours after the administration of cisplatin, all the animals were anaesthetized with diethyl ether and rapidly decapitated and submitted to micronucleus test and biochemical and histological analysis.
Determination of serum biochemical parameters
Blood samples were kept at room temperature for 1 h and then centrifuged at 3000 rpm for 30 min to obtain serum. Blood urea nitrogen (BUN) and serum creatinine were measured spectrophotometrically, using commercially available kits (Merck, India), according to the manufacturer's instructions.
Micronucleus test
Genotoxic effects were evaluated in the mouse bone marrow by the micronucleus test (Schmid, Citation1975; Jagetia & Jacob, Citation1992). The femurs of each animal were dissected, out and the bone marrow was flushed out into Dulbecco's modified Eagle's medium (DMEM). The suspension was centrifuged. A few drops of fetal calf serum (FCS) were added, and the pellet was mixed thoroughly. Smears were drawn onto precleaned coded slides using a drop of the resultant suspension in FCS. The slides were air-dried and fixed in absolute methanol. The slides were then stained with 0.125% acridine orange in Sorensen's buffer of pH 6.8 and washed twice in Sorensen's buffer. The slides mounted in Sorensen's buffer were observed under × 400 magnification using a fluorescent microscope (Helmut Hund, Wetzlar, Germany). A minimum of 1000 polychromatic erythrocytes (PCE) were counted for the presence of micronuclei (MN) for each animal. A total of not less than 6000 PCE were counted for each drug dose. The MN was then recorded and MN per thousand PCE was calculated.
Histological methods
Small pieces of the cortex of the left kidney of each animal were fixed in 10% neutral buffered formalin, dehydrated in graded alcohol, and embedded in paraffin wax. Sections of 5-µm thickness were stained with hematoxylin and eosin (H&E) and subjected to microscopic examination for the presence of glomerular congestion, tubular casts, peritubular congestion, epithelial desquamation, blood vessel congestion, interstitial edema, and inflammatory cells.
Determination of free radical scavenging activity
Scavenging effect on DPPH radical
The effect of the extracts on DPPH radical was estimated according to the procedure given elsewhere (Moure et al., Citation2000). Two milliliters of a 3.6 × 10−5 M methanol solution of DPPH were added to 50 µl of a methanol solution (1 mg/ml) of the antioxidant. The decrease in the absorbance at 515 nm was continuously recorded in a Shimadzu UV-160A spectrophotometer (Shimadzu, Kyoto, Japan) for 16-min at room temperature. The scavenging effect (decrease of absorbance at 515 nm) was plotted against time, and the percentage of DPPH radical scavenging ability of the sample was calculated from the absorbance value at the end of 16 min duration using the following equation:
Measurement of antioxidative ability by the DMPD method
The N.,N.-dimethyl-p.-phenlylenediamine (DMPD) method (Fogliano et al., Citation1999) was used to determine the antioxidant activity. PME extract (50 µl) was added to 1 ml of a solution containing the DMPD radical cation in acetate buffer. The quenching of absorbance at 505 nm was compared with that obtained by a standard solution (1 mg/ml) of ascorbic acid and silymarin. The percentage of the absorbance of uninhibited radical cation solution (blank) was calculated using the following equation;
where A0 is the absorbance of uninhibited radical cation and Af is the absorbance measured at 10 min after the addition of antioxidant samples.
Statistical analysis
Values are expressed as mean ± S.E.M. Comparisons among the groups were tested by one-way ANOVA using Graph Pad Prism, version 4.0 (Graph Pad Software, San Diego, CA, USA). When the p value obtained from ANOVA was significant (p < 0.05), the Tukey test was applied to test for differences among groups.
Results
Phytochemical screening
Preliminary phytochemical screening (Harborne, Citation1984) revealed the presence of carbohydrates, steroids, glycosides, terpenoids, flavanoids, and tannins.
Biochemical parameters
lists the effects of oral administration of PME on cisplatin-induced elevation of BUN, serum creatinine, and the kidney to body weight ratio. The results reveal that the levels of BUN and serum creatinine show significant increase in cisplatin-treated animals (110.19 and 2.00 mg/dl compared with 28.33 and 0.34 for control animals). PME at 400 and 600 mg/kg body weight, however, reverses the increase of BUN and serum creatinine levels in cisplatin-treated animals. Similar results were also obtained for kidney to body weight ratio in a dose-dependent manner. When PME at 600 mg/kg was given orally for 7 days before administration of cisplatin, the level of BUN increased to 48.99 ± 4.63 mg/dl compared with 110.19 ± 12.81 mg/dl for animals given cisplatin alone (control = 28.33 ± 1.84 mg/dl), amounting to 74% protection. Serum creatinine levels increased from 0.34 mg/dl in the control to 2.00 mg/dl on treatment with cisplatin. When PME at 600 mg/kg body weight was given, serum creatinine increased to 0.93 mg/dl amounting to approximately 64% protection. The protective effect of PME on cisplatin-induced nephrotoxicity is thus dose-dependent. Further increase in the dose did not lead to improved protection (data not shown).
Table 1 Effect of Phyllanthus maderaspatensis. Linn (PME) on cisplatin-induced renal damage.
Micronucleus assay
shows the frequencies of micronucleuted polychromatic erythrocytes (MNPCEs) in the bone marrow cells of mice pretreated with PME before exposure to cisplatin. Development of micronuclei (MN) was observed in bone marrow cells after cisplatin. The incidence of MN was found to be more in polychromatic erythrocytes in cisplatin-treated mice compared with control animals. PME at the dose levels of 400 and 600 mg/kg body weight were thus effective in exerting significant antigenotoxic effects against cisplatin.
Table 2 Effect of Phyllanthus maderaspatensis. Linn (PME) on frequency of micronucleus in bone marrow cells of mice.
Histopathological studies
shows the effect of PME on histological features of cisplatin-induced renal damage. The results reveal that cisplatin-treated mice show a marked congestion of glomeruli with numerous tubular casts associated with epithelial desquamation as compared with the control animals. Marked peritubular congestion and edema were also observed. The interstitium showed infiltration with inflammatory cells and congestion. These features suggest that cisplatin induces acute tubular necrosis. Whereas groups 4 and 5 continued to show glomerular and peritubular congestion with tubular casts and inflammatory cells, group 6 (PME 600 mg/kg body weight) showed complete normalization of kidney section. Mild glomerular, peritubular congestion and inflammatory cells were, however, observed in group 6.
Table 3 Effect of Phyllanthus maderaspatensis. Linn (PME) on histological features of cisplatin-induced renal damage.
Antioxidant studies
Scavenging effect on DPPH radical
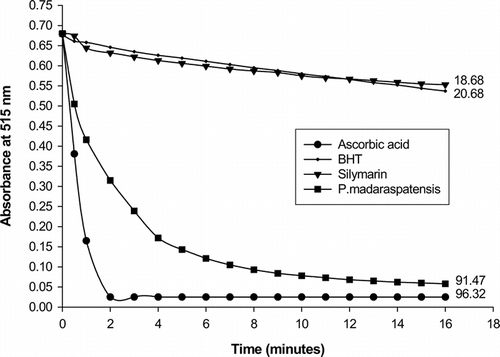
The radical scavenging activity using a DPPH-generated radical was tested for PME along with ascorbic acid, BHT, and silymarin, and the results are shown in . The results reveal that the radical scavenging ability of PME is almost equal to ascorbic acid but BHT and silymarin show very slow kinetic behavior. In terms of percentage, the inhibiting activity (at 16 min) is in the order: ascorbic acid (96.32%), PME (91.47%), BHT (20.68%), and silymarin (18.68%).
Measurement of antioxidative ability by the DMPD method
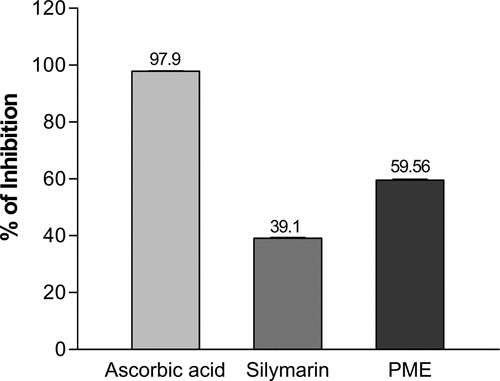
The antioxidant ability of PME, ascorbic acid, and silymarin was measured by using the DMPD method as described under “materials and Methods”. The results are shown in . Ethanol extract of PME produces about 60% inhibition of DMPD radical at 505 nm.
Discussion
Cisplatin is an important antineoplastic agent useful in treating many types of solid tumors. Its use, however, is limited by its nephro- and genotoxicity. Although the mechanism of the nephro- and genotoxicity is not clear, oxygen free radicals have been implicated by earlier workers. A relationship between oxidative stress and nephrotoxicity has been well demonstrated in many experimental animal models (Li et al., Citation1995; Devipriya & Shyamaladevi, Citation1999). Numerous in vivo. and in vitro. studies have demonstrated that reactive oxygen metabolites, such as free radical species, superoxide, hydroxyl radical anion, and hydrogen peroxide are important mediators of tissue injury (Weiss & LoBuglio, Citation1982; Fox, Citation1984; Varani et al., Citation1985; Fantone & Ward, Citation1982). Oxygen free radicals have also been implicated in several biological processes, potentially important in glomerular diseases (Shah et al., Citation1984Citation1987). Previous reports suggest that cisplatin induces nephrotoxicity by initiation of lipid peroxidation and depletion of cellular thiols (Zhang & Lindup, Citation1994; Rice-Evans & Miller, Citation1995). Cisplatin has also been shown to inhibit the activity of antioxidant enzymes (superoxide dismutase, catalase, and glutathione peroxidase) in rat kidneys (Sdzuka et al., Citation1992). All these suggest that cisplatin cytotoxicity results from generation of reactive oxygen species.
It is known that chemoprotective agents are capable of exerting their antigenotoxic effects by one or a combination of mechanisms such as inhibiting formation of reactive carcinogenic metabolites, induction of enzymes that detoxify carcinogens, scavenging reactive oxygen species, and influencing apoptosis and inhibiting cell proliferation (Sharma et al., Citation1994; Wattenberg, Citation1985). Further, flavonoids are known to be potent antioxidants capable of modulating the activities of various enzyme systems due to their interaction with biomolecules (Devipriya & Shyamaladevi, Citation1999). Our preliminary phytochemical studies reveal that PME contains a number of flavanoids and tannins. The results of in vitro. antioxidant studies carried out also reveal that PME may contain powerful inhibitor compounds that might act as primary antioxidants that react with free radicals.
The aim of the current study was to investigate the possible role of PME in modulating the in vivo. nephro- and genotoxicity of cisplatin use in cancer chemotherapy. Results from the current investigation demonstrate that cisplatin induces nephro- and genotoxicity as evidenced by elevated blood urea nitrogen and serum creatinine and an increase in the frequency of MNPCEs, as well as in the observations made in the histopathological features of acute tubular necrosis. The alcohol extract of PME when administered at 400 and 600 mg/kg body weight for seven days, 24 h after cisplatin treatment, thus shows a significant reduction in the increase in blood urea nitrogen, serum creatinine, and the frequency of MNPCEs in the bone marrow cells. A marked recovery in the kidneys is also seen microscopically. The results of the current study show that the ethanol extract of Phyllanthus maderaspatensis. possess marked chemoprotective activity via its antioxidant potential and may have a promising role to play in the treatment of cisplatin-induced nephro- and genotoxicity. Further work, such as evaluating the chemoprotective role of this plant in chronic models and isolation of active compounds, is in progress.
Acknowledgments
We thank Dr. A. Amit, of Natural Remedies Pvt. Ltd., Bangalore, for providing plant material. Praveen Bommu thanks the Department of Science and Technology, Government of India, New Delhi, India, for the award of a Junior Research Fellowship.
References
- Asha VV, Pushpangadan P (1998): Preliminary evaluation of the antihepatotoxic activity of Phyllanthus kozhikodianus., Phyllanthus maderspatensis. and Solanum indicum.. Fitoterapia 59: 255–259, [CSA]
- Asha VV, Akhila S, Wills PJ, Subramoniam A (2004): Further studies on the antihepatotoxic activity of Phyllanthus maderaspatensis. Linn. J Ethnopharmacol 92: 67–70, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Chu G (1994): Cellular responses to cisplatin. J Biol Chem 269: 787–790, [PUBMED], [INFOTRIEVE], [CSA]
- Devipriya S, Shyamaladevi CS (1999): Protective effect of quercetin in cisplatin induced cell injury in the rat kidney. Indian J Pharmacol 31: 422, [CSA]
- Fantone JC, Ward PA (1982): Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol 107: 395–418, [PUBMED], [INFOTRIEVE], [CSA]
- Fogliano V, Verde V, Randazzo G, Ritieni A (1999): Method for measuring antioxidant capacity of wines. J Agric Food Chem 47: 1035–1040, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Fox RB (1984): Prevention of granulocyte-mediated oxidative injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J Clin Invest 74: 1456–1464, [PUBMED], [INFOTRIEVE], [CSA]
- Harborne JB (1984): Phytochemical Methods. New York, Chapman and Hall, pp. 123–175.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Human: (1987): In: Overall Evaluation of Carcinogenicity, Vol. 42. Lyon, France, International Agency for Research on Cancer.
- Jagetia GC, Jacob PS (1992): Vinblastine treatment induces dose-dependent increases in the frequency of micronuclei in mouse bone marrow. Mutat Res 280: 87–92, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Kirthikar KR, Basu BD (1999): In: Blatter E, Calus JF, Mhaskar KS, eds., Indian Medicinal Plants. Dehradun, India, International Book Distributors, pp. 2222–2223.
- Kociba RJ, Sleight SD, Rosenberg B (1970): Inhibition of Dunning ascitic leukemia and Walker-256 carcinosarcoma with cis.-diamminedichloroplatinum (NSC-119875). Cancer Chemother Rep 54: 325–328, [PUBMED], [INFOTRIEVE], [CSA]
- Li Q, Bowmer CJ, Yates MS (1995): Amelioration of cisplatin nephrotoxicity with glycine: Dose dependency in rats. J Pharm Pharmacol 47: 223–226, [PUBMED], [INFOTRIEVE], [CSA]
- Loehrer PJ, Einhorn LH (1984): Drugs five years later. Cisplatin. Ann Intern Med 100: 704–713, [PUBMED], [INFOTRIEVE], [CSA]
- Masuda H, Tanaka T, Takahama U (1994): Cisplatin generates superoxide anion by interaction with DNA in a cell-free system. Biochem Biophys Res Commun 203: 1175–1180, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Moure A, Franco D, Sineiro J, Dominguez H, Nunez MJ, Lema JM (2000): Evaluation of extracts from Gevuina avellana. hulls as antioxidants. J Agric Food Chem 48: 3890–3897, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Pinto AL, Lippard SJ (1985): Binding of the antitumor drug. cis.-diamminedichloroplatinum II cisplatin to DNA. Biochim Biophys Acta 780: 167–180, [PUBMED], [INFOTRIEVE], [CSA]
- Prasad SB, Giri A (1994): Antitumor effect of cisplatin against murine ascites Dalton's lymphoma. Indian J Exp Biol 32: 57–62, [CSA]
- Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham IB (1995): The relative antioxidant activities of plant-derived polyphenolic flavanoids. Free Radic Res 22: 375–383, [PUBMED], [INFOTRIEVE], [CSA]
- Rosenberg B (1985). Fundamental studies with cisplatin. Cancer 55: 2303–2316, [PUBMED], [INFOTRIEVE], [CSA]
- Schmidt W (1975). The micronucleus test. Mutat Res 31: 9–15, [CSA]
- Sdzuka Y, Shoji T, Takino Y (1992): Effect of cisplatin on the activation of enzymes which protect against lipid peroxidation. Biochem Pharmacol 43: 1872–1875, [CROSSREF], [CSA]
- Shah SV (1984): Effect of enzymatically generated reactive oxygen metabolites on the cyclic nucleotide content in isolated glomeruli. J Clin Invest 74: 393–401, [PUBMED], [INFOTRIEVE], [CSA]
- Shah SV, Barcos WH, Basci A (1987): Degradation of human glomerular basement membrane by stimulated neutrophils. Activation of a metalloproteinase by reactive oxygen metabolite. J Clin Invest 79: 25–31, [PUBMED], [INFOTRIEVE], [CSA]
- Sharma S, Stutzman JD, Kelloff GJ, Steele VE (1994): Screening of potential chemopreventive agents using biochemical markers of carcinogenesis. Cancer Res 54: 5848–5855, [PUBMED], [INFOTRIEVE], [CSA]
- Sokmen A, Jones BM, Erturk M (1999): The in vitro. antibacterial activity of Turkish medicinal plants. J Ethnopharmacol 67: 79–86, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Sorsa M, Hemminki K, Vainio H (1985): Occupational exposure to anti-cancer drugs: potential and hazard. Mutat Res 154: 135–149, [PUBMED], [INFOTRIEVE], [CSA]
- Surh YJ (1999): Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res 428: 305–327, [PUBMED], [INFOTRIEVE], [CSA]
- Thyagarajan SP, Subramanian S, Thirunalasundari T, Venkateswaran PS, Blumberg BS (1988): Effect of Phyllanthus amarus. on chronic carriers of hepatitis B virus. Lancet 2: 764–766, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Unander DW, Venkateswaran PS, Millman I, Bryan HH, Blumberg BS (1990): Phyllanthus. species: Source of new antiviral compounds. In: Janick J, Simon JE, eds., Advances in New Crops. Portland, Timber Press, pp. 518–521.
- Unander DW, Webster GL, Blumberg BS (1995): Usage and bioassays in phyllanthus. (Euphorbiaceae). IV. Clustering of antiviral uses and other effects. J Ethnopharmacol 45: 1–18, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Varani J, Fligiel SE, Till GO, Kunkel RG, Ryan US, Ward PA (1985): Pulmonary endothelial cell killing by human neutrophils. Possible involvement of hydroxyl radical. Lab Invest 53: 656–663, [PUBMED], [INFOTRIEVE], [CSA]
- Wattenberg LW (1985): Chemoprevention of cancer. Cancer Res 45: 1–8, [PUBMED], [INFOTRIEVE], [CSA]
- Weijl NI, Cleton FJ, Osanto S (1997): Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat Rev 23: 209–240, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Weiss SJ, LoBuglio AF (1982): Phagocyte-generated oxygen metabolites and cell injury. Lab Invest 47: 5–18, [PUBMED], [INFOTRIEVE], [CSA]
- Zamble DB, Lippard SJ (1995): Cisplatin and DNA repair in cancer chemotherapy. Trends Biochem Sci 20: 435–439, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Zhang JG, Lindup WE (1994): Cisplatin nephrotoxicity: Decreases in mitochondrial protein sulfhydryl concentration and calcium uptake by mitochondria from rat renal cortical slices. Biochem Pharmacol 47: 1127–1135, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]