Abstract
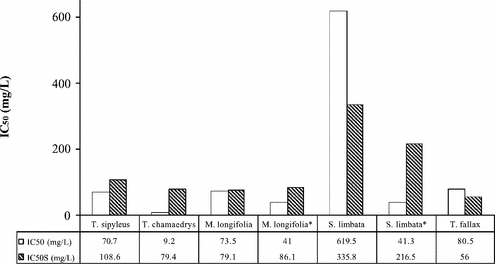
In this study, antioxidant properties of Thymus sipyleus. Boiss. subsp. sipyleus. var. sipyleus., Teucrium chamaedrys L.., Mentha longifolia. (L.) Hudson subsp. longifolia., Salvia limbata. C.A. Meyer, and Thymus fallax. Fisch. & Mey. were investigated. Antioxidant and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activities, reducing powers, and the amount of total phenolic compounds of the extracts were studied. The highest antioxidant activity was shown by T. chamaedrys. (decoction, IC50: 9.2 µg/ml), and the lowest one was S. limbata. (decoction, IC50: 619.5 µg/ml). The highest DPPH radical scavenging activity was shown by T. fallax. [decoction, IC50S: 56 µg/ml (IC50S is the extract concentration (µg/ml) required for 50% inhibition of the DPPH solution absorbance at 517 nm)] while the lowest one was S. limbata. (decoction, IC50S: 335.8 µg/ml). The highest reducing power and amount of total phenolic compounds was shown by T. chamaedrys. (decoction, 29.9 µg/ml ascorbic acid equivalent, 27.9 µg/ml gallic acid equivalent, respectively), and the lowest one was S. limbata. (decoction, 5.1 µg/ml ascorbic acid equivalent, 9.9 µg/ml gallic acid equivalent, respectively) at 250 µg/ml extract concentration.
Introduction
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are various forms of activated oxygen and nitrogen, which include free radicals such as superoxide ions (O2⋅−), hydroxyl (OH⋅) and nitric oxide radicals (NO⋅), as well as non-free-radical species such as hydrogen peroxide (H2O2), and nitrous acid (HNO2) (Halliwell, Citation1995; Halliwell & Aruoma, Citation1997; Squadriato & Peyor, Citation1998). In living organisms. ROS and RNS can form in different ways. Normal aerobic respiration, stimulated polymorphonuclear leukocytes and macrophages, and peroxisomes appear to be the main endogenous sources of most of the oxidants produced by cells (Fridovich, Citation1986; Halliwell, Citation1994; Alho & Leinonen, Citation1999). Exogenous sources of free radicals include tobacco smoke, ionizing radiation, organic solvents, and pesticides (Halliwell & Gutteridge, Citation1989; Davies, Citation1995; Papas, Citation1996; Robinson et al., Citation1997). Free radicals can cause lipid peroxidation in foods that leads to their deterioration (Sasaki et al., Citation1996). ROS and RNS may cause DNA damage that could lead to mutation (Aruoma, Citation1998; Sawa et al., Citation1999). In addition, ROS and RNS have been implicated in more than 100 diseases, including malaria, acquired immunodeficiency syndrome, heart disease, stroke, arteriosclerosis, diabetes, and cancer (Tanizawa et al., Citation1992; Hertog et al., Citation1993; Duh, Citation1998; Alho & Leinonen, Citation1999). When produced in excess, ROS can cause tissue injury, while, tissue injury can itself cause ROS generation (Aruoma, Citation1998). Nevertheless, all aerobic organisms, including human beings, have antioxidant defenses that protect against oxidative damage and numerous damage removal and repair enzymes to remove or repair damaged molecules (Davies, Citation1995; Fridovich, Citation1995; Granelli et al., Citation1995; Sun et al., Citation1998). However, the natural antioxidant mechanisms can be inefficient, hence dietary intake of antioxidant compounds becomes important (Halliwell, Citation1994; Terao et al., Citation1994; Duh, Citation1998; Espin et al., Citation2000). Although there are some synthetic antioxidant compounds, such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), that are commonly used in processed foods, it has been reported that these compounds may have side effects (Branien, Citation1975; Yamamoto et al., Citation1980; Witschi, Citation1981; Takahashi et al., Citation1985; Lindenschmidt et al., Citation1986; Patterson et al., Citation1987; Kehrer & DiGiovanni, Citation1990). In addition, it has been suggested that there is an inverse relationship between dietary intake of antioxidant-rich foods and the incidence of a number of human diseases (Rice-Evans et al., Citation1997; Lu & Foo, Citation2000). Therefore, research into the determination of natural antioxidant sources is important.
In this study, we have determined the antioxidant and DPPH radical scavenging activities (AA and DPPH-RS), reducing powers (RP), and amount of phenolic compounds (APC) of some medicinal Lamiaceae plants that have been used especially in Eastern Turkey.
Materials and Methods
Collection of plants and identification
Five Lamiaceae species were collected in July–August in villages of Ilιca District: Thymus sipyleus. subsp. sipyleus. var. sipyleus. (“kekik otu”), Teucrium chamaedrys. (“basur otu”), Mentha longifolia. subsp. longifolia. (“yarpuz”), Salvia limbata. (“kedi kuyruğu”), and Thymus fallax. (“kekik otu”). Their local names, parts used, uses/ailments treated, preparations, and extraction solvents are given in Tables and . They were authenticated by Dr. Ufuk Özgen and Dr. Yusuf Kaya. Voucher specimens were deposited at Ankara Üniversitesi Eczacιlιk Fakültesi Herbaryumu (AEF) and Atatürk Üniversitesi Fen Fakültesi Herbaryumu (ATA): Mentha longifolia. (L.) Hudson subsp. longifolia. (AEF 21171), Salvia limbata. C.A. Meyer (AEF 21163), Teucrium chamaedrys. L. (AEF 21179), Thymus fallax. Fisch. & Mey. (AEF 21199), and Thymus sipyleus. Boiss. subsp. sipyleus. var. sipyleus. (ATA 9718).
Table 1 Local names, parts used, uses/ailments treated, and preparations of some Lamiaceae plants used by the villagers of Ilιca District, Erzurum, Turkey.
Table 2 Localities, parts, and extraction solvents of plants used in activity studies.
Preparation of extract solutions
Taking into consideration traditional usage in general, the most suitable parts of plants and extraction solvents were chosen. Plants that have been used generally as decoctions by the public were extracted with water. Plants that have been employed generally for other usages (eating, powdering for treatment) were also extracted with methanol. All plants were dried and powdered using a mill before extraction.
For extraction, 20 g of powdered sample was extracted with 400 ml water or methanol by reflux for about one-half hour and then filtered. Extracts were evaporated and then lyophilized. Water extracts of M. longifolia., T. chamaedrys., T. fallax., T. sipyleus., and also methanol extracts of M. longifolia. and S. limbata. were tested. In the experiments, different concentrations of all extracts were studied and results of 50, 100, 250, and 500 µg/ml concentrations are given in .
Table 3 Antioxidant activities of plant extracts.
Antioxidant activity (thiobarbituric acid test)
The in vitro. antioxidant activity tests were carried out using the lipid peroxidation of liposomes assay where the thiobarbitoric acid (TBA) test has been applied to assess the efficacy of the compounds to protect liposomes from lipid peroxidation (Fernàndez et al., Citation1997). The TBA reaction is based on the fact that peroxidation of most membrane systems leads to formation of small amounts of free malonaldehyde (MDA). One molecule of MDA reacts with two molecules of TBA to yield a colored product, which in an acid environment absorbs light at 532 nm, and it is readily extractable into organic solvents. It can, thus, be measured and quantified spectrophotometrically. The intensity of color is a measure of MDA concentration. Absorbance at 532 nm was determined on a Helios β. UV/Vis spectrophotometer (England).
The presence of any antioxidant compound in the lipid peroxidation assay reaction mixture will lead to a reduction of the extent of peroxidation. The methanolic and/or aqueous extracts from investigated plants were tested for their antioxidant activity against liposomes that were prepared from bovine brain extract in phosphate-buffered saline (5 mg/ml) (Jacobi et al., Citation1999). Peroxidation was started by adding FeCl3 and ascorbic acid followed by incubation at 37°C for 20 min. Ascorbic acid is a well-known antioxidant but also exhibits pro-oxidant properties in the presence of certain transition metal ions, such as Fe or Cu. BHT in ethanol was added to prevent lipid peroxidation during the TBA test itself. Propyl gallate was used as a positive control. Data are given as % peroxidation inhibition-concentration graphic and IC50 (mg/l extract concentration required for 50% peroxidation inhibition).
DPPH radical scavenging
DPPH radical scavenging was carried out according to the Blois method (Blois, Citation1958) with a slight modification. Briefly, a 1 mM solution of DPPH radical solution in methanol was prepared and then 1 ml of this solution was mixed with 3 ml of extract solution in ethanol. Final concentrations of extracts were 50, 100, 250, and 500 µg/ml. After 30 min incubation in the dark, absorbance was measured at 517 nm. This activity is given as IC50S. IC50S is the extract concentration (μg/ml) required for 50% inhibition of the DPPH radical absorbance at 517 nm). The % DPPH radical scavenging that is calculated in the equation:
The control was the test solution without extract.
Reducing power
Measurement of the reducing power was carried out as described previously (Yιldιrιm et al., 2001). Briefly, the lyophilized extracts in 1 ml of corresponding solvents were mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml potassium ferricyanide [K3Fe(CN)6] (1%), then the mixture was incubated at 50°C for 30 min. Afterward, 2.5 ml of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. Finally, 2.5 ml of the supernatant was mixed with 2.5 ml distilled water and 0.5 ml FeCl3 (0.1%), and the absorbance was measured at 700 nm. Final concentrations of extracts were 50, 100, 250, and 500 µg/ml. Data were presented as ascorbic acid equivalents (Asc. Acid Eq.) using a calibration curve.
Amount of total phenolic compounds
The determination of total phenolic compounds was carried out as described previously (Singleton et al., Citation1999). Briefly, extract solutions were transferred into test tubes, then final volumes were adjusted to 4 ml by addition of distilled water. Afterward, 0.25 ml of Folin-Ciocalteu reactive (FCR) (Fluka, Switzerland) was added to the mixture, and after 3 min, 0.75 ml of Na2CO3 (20%) was added. Final concentrations of extracts were 50, 100, 250, and 500 µg/ml. Subsequently, the mixtures were shaken on a shaker for 2 h at room temperature, and then the absorbance was measured at 760 nm. Gallic acid was used as a standard. The phenolic compound content was determined as the gallic acid equivalent using a calibration curve.
Statistical analysis
Each of the experiments were performed in triplicate. The results shown are the means of these measurements (). Data were analyzed with SPSS software to determine whether there was any correlation between antioxidant properties in an extract. Pearson parametric correlation analysis was carried out using SPSS software.
Results and Discussion
All of the extracts examined exhibited antioxidant properties. The most effective antioxidant potential was shown by T. chamaedrys., and the least effective was the aqueous extract of S. limbata. ( and ).
Data were analyzed with SPSS software to determine whether a correlation between antioxidant properties in an extract exist (). From the table, we can suggest that AA may be affected by different parameters, such as DPPH-RS, RP, and APC. Effects of these parameters are changeable. For example, there is a correlation between AA and DPPH-RS in some extracts but not others. However, there are statistically significant correlations between reducing powers and amount of phenolic compounds in all of the extracts. We found similar results in a previous study (Özgen et al., Citation2004).
Table 4 p values of correlation analyses.
The Lamiaceae family consists of approximately 200 genera of cosmopolitan distribution, many of them of economic importance due to essential oil production. Most genera of the Lamiaceae are thus rich sources of terpenoids and also they contain a considerable amount of various iridoid glycosides, flavonoids, and phenolic compounds. Within this family, the flavonoids are predominantly 6- and/or 8-substituted flavone derivatives, and flavonols are rarely encountered (Valant-Vetschera, Citation2003).
It has been shown that the aerial parts of M. longifolia. contain flavones and their glycosides (luteolin 7-glucoside, luteolin 7-rutinoside, luteolin 7-glucuronide, apigenin 7-glucuronide, acacetin 7-rutinoside, diosmetin 7-rutinoside, hesperetin 7-rutinoside, and eriodictyol 7-rutinoside) and phenolic acids (Bourwieg & Pohl, Citation1973; Mimica-Dukic et al., Citation1996Citation1999; Sharaf et al., Citation1999; Ghoulami et al., Citation2001; Jahan et al., Citation2001; Ali et al., Citation2002).
Salvia. species are widely used in traditional medicine and are a rich source of flavonoids and phenolic acids. Flavones, flavonols, and their glycosides that are present in Salvia. constitute the majority of flavonoids. Anthocyanins are abundant in red to blue Salvia. flowers, whereas the presence of proanthocyanidins (condensed tannins) in Salvia. has not been demonstrated conclusively (Lu & Foo, Citation2002). S. limbata. also contains norsesterter-penes and diterpenes (Ulubelen et al., Citation1996).
T. chamaedrys. contains neoclerodane diterpenoids, steroidal compounds, phenylpropanoid glycosides (teucrioside and poliumoside), 3,4-dihydroxyphenylethanoid glucoside (acteoside), flavonoids (cirsimaritin-5,4-OH-6,7-OMe-flavone-, cirsiliol-5,3′,4′-OH-6,7-OMe-flavone-, and luteolin-5,7,3′,4′-OH-flavone-) and phenolic acid (6-OH caffeic acid) (Kouzi et al., Citation1994; Ulubelen et al., Citation1994; Calis et al., Citation1996; Bedir & Calis, Citation1997; Pedersen, Citation2000; Valant-Vetschera, Citation2003).
Thymus. genus contains flavonoids (luteolin, apigenin, eryodictiol, naringenin, diosmetin, and their glycosides) and phenolic acids (rosmarinic acid, 6-hydroxyrosmarinic acid, caffeic acid, 6-hydroxycaffeic acid, protocatechuic acid, chlorogenic acid, syringic acid, p.-coumaric acid, 3,5-dicaffeoylquinic acid, gentisic acid, p.-hydroxybenzoic acid, vanillic acid, and p.-coumaric acid) (Voirin et al., Citation1985; Miura & Nakatani, Citation1989; Blazquez et al., Citation1994; Pedersen, Citation2000; Zgorka & Glowniak, Citation2001). There are so far no reports on flavonoids and phenolic acids associated with T. sipyleus. and T. fallax..
As mentioned above, these plants contain phenolic compounds, especially flavonoids. It is well-known that flavonoids possess a wide range of antioxidant activities. These antioxidant properties are based on their phenolic structures (Hall & Cuppett, Citation1997). Phenolic compounds are also thought to be capable of regenerating endogenous α -tocopherol, in the phospholipid bilayer of lipoprotein particles, back to its active antioxidant form. They are also known to inhibit various types of oxidizing enzymes. These potential mechanisms of antioxidant action make the diverse group of phenolic compounds an interesting target in the search for health-beneficial phytochemicals (Halliwell & Gutteridge, Citation1989; Hall & Cuppett, Citation1997). It has been reported that rosmarinic acid is the major component present in Lamiaceae plants, and it possesses a potent antioxidant activity. Thus, the observed antioxidant properties of Lamiaceae plants are presumably strongly depend on rosmarinic acid (Hall & Cuppett, Citation1997).
Acknowledgment
The authors would like to thank Atatürk University Rectorate for financial support (grant 2001/137 University Research Fund).
References
- Alho H, Leinonen J (1999): Total antioxidant activity measured by chemiluminescence method. Methods Enzymol 299: 3–15, [PUBMED], [INFOTRIEVE], [CSA]
- Ali MS, Saleem M, Ahmad W, Parvez M, Yamdagni R (2002): A chlorinated monoterpene ketone, acylated β.-sitosterol glycosides and a flavanone glycoside from Mentha longifolia. (Lamiaceae). Phytochemistry 59: 889–895, [CROSSREF], [CSA]
- Aruoma OI (1998): Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc 75: 199–212, [CSA]
- Bedir E, Calis I (1997): Teucrium polium. ve Teucrium chamaedrys. subsp. syspirense' den elde edilen fenilpropanoit heterozitleri.. Hacettepe Univ Eczacilik Fak Derg 17: 9–16, [CSA]
- Blazquez MA, Manez S, Zafrapolo MC (1994): Further flavonoids and other phenolics of Thymus webbianus.. Z Naturforsch C 49: 687–688, [CSA]
- Blois MS (1958): Antioxidant determinations by the use of a stable free radical. Nature 181: 1198–1200, [CSA]
- Bourwieg D, Pohl R (1973): Flavonoids from Mentha longifolia.. Planta Med 24: 304–314, [PUBMED], [INFOTRIEVE], [CSA]
- Branien AL (1975): Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. J Am Oil Chem Soc 52: 59–63, [CSA]
- Calis I, Bedir E, Wright AD, Sticher O (1996): Neoclerodane diterpenoids from Teucrium chamaedrys. subsp syspirense.. J Nat Prod 59: 457–460, [CROSSREF], [CSA]
- Davies KJA (1995): Oxidative stress the paradox of aerobic life. Biochem Symp 61: 1–31, [CSA]
- Duh PD (1998): Antioxidant activity of Burdock: Its scavenging effect on free-radical and active oxygen. J Am Oil Chem Soc 75: 455–461, [CSA]
- Espin JC, Soler-Rivas C, Wichers HJ (2000): Characterization of the total free radical scavenging capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J Agr Food Chem 48: 648–656, [CROSSREF], [CSA]
- Fernàndez J, Pérez-Alvarez J, Fernàndez-Lopez J (1997): Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem 59: 345–353, [CROSSREF], [CSA]
- Fridovich I (1986): Superoxide dismutases. Methods Enzymol 58: 61–97, [CSA]
- Fridovich I (1995): Superoxide radical and superoxide dismutases. Annu Rev Biochem 64: 97–112, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Ghoulami S, Il Idrissi A, Fkih-Tetouani S (2001): Phytochemical study of Mentha longifolia. of Morocco. Fitoterapia 72: 596–598, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Granelli K, Björck L, Appelqvist LA (1995): The variation of SOD and XO activities in milk using an improved method to quantitate SOD activity. J Sci Food Agr 67: 85–91, [CSA]
- Hall CA, Cuppett SL (1997): Structure-activities of natural antioxidants. In: Aruoma OI, Cuppett SL, eds., Antioxidant Methodology: In vivo and in vitro Concepts. Champaign, IL, AOCS Press, pp. 141–172.
- Halliwell B (1994): Free radicals antioxidants and human disease: Curiosity, cause or consequence. Lancet 344: 721–724, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Halliwell B (1995): How to characterize an antioxidant: An update. Biochem Soc Symp 61: 73–101, [PUBMED], [INFOTRIEVE], [CSA]
- Halliwell B, Aruoma OI (1997): Free radicals and antioxidants: The need for in vivo markers of oxidative stress. In: Aruoma OI, Cuppett SL, eds., Antioxidant Methodology: In vivo and in vitro Concepts. Champaign, IL: AOCS Press, pp. 1–22.
- Halliwell B, Gutteridge JM (1989): Free Radicals in Biology and Medicine, 2nd ed. Oxford, Clarendon Press.
- Hertog MGL, Feskens EJM, Hollman PCH, Katan MB, Kromhout D (1993): Dietary antioxidant flavonoids and risk of coronary heart disease: The zutphen elderly study. Lancet 342: 1007–1014, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Jacobi H, Hinrichen M, Web D, Witte I (1999): Induction of lipid peroxidation in human fibroblasts by the antioxidant propyl gallate in combination with copper (II). Toxicol Lett 110: 183–190, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Jahan N, Malik A, Muhammad P (2001): New flavonoid from Mentha longifolia Heterocycles. 55: 1951–1955, [CSA]
- Kehrer JP, DiGiovanni J (1990): Comparison of lung injury induced in 4 strains of mice by butylated hydroxytoluene. Toxicol Lett 52: 55–61, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Kouzi SA, Mcmurtry RJ, Nelson SD (1994): Hepatotoxicity of germander (Teucrium chamaedrys. L.) and one of its constituent neoclerodane diterpenes teucrin A in the mouse. Chem Res Toxicol 7: 850–856, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Lindenschmidt RC, Tryka AF, Goad ME, Witschi HP (1986): The effects of dietary butylated hydroxytoluene on liver and colon tumor development in mice. Toxicology 38: 151–160, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Lu Y, Foo LY (2000): Antioxidant radical scavenging activities of polyphenols from apple pomace. Food Chem 68: 81–85, [CROSSREF], [CSA]
- Lu Y, Foo LY (2002): Polyphenolics of Salvia.. Phytochemistry 59: 117–140, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Mimica-Dukic N, Jakovljevic V, Mira P, Gasic O, Szabo A (1996): Pharmacological study of Mentha longifolia. phenolic extracts. Pharm Biol 34: 359–364, [CSA]
- Mimica-Dukic N, Popovic M, Jakovljevic V, Szabo A, Gasic O (1999): Pharmacological Studies of Mentha longifolia. phenolic extracts. II. Hepatoprotective activity. Pharm Biol 37: 221–224, [CSA]
- Miura K, Nakatani N (1989): Antioxidative activity of flavonoids from thyme (Thymus vulgaris. L.). Agric Biol Chem 53: 3043–3045, [CSA]
- Özgen U, Mavi A, Terzi Z, Coşkun M, Yιldιrιm A (2004): Antioxidant properties of some Asteraceae (Compositae) species. Turkish J Pharm Sci 1: 203–216, [CSA]
- Papas AM (1996): Determinants of antioxidant status in human. Lipids 31: 77–82, [CSA]
- Patterson RM, Keith LA, Stewart J (1987): Increase in chromosomal abnormalities in Chinese hamster ovary cells treated with butylated hydroxytoluene in vitro. Toxicol in Vitro 1: 55–57, [CROSSREF], [CSA]
- Pedersen JA (2000): Distribution and taxonomic implications of some phenolics in the family Lamiaceae determined by ESR spectroscopy. Biochem Syst Ecol 28: 229–253, [CROSSREF], [CSA]
- Rice-Evans CA, Sampson J, Bramley PM, Hollowa DE (1997): Why do we expect carotenoids to be antioxidants in vivo. Free Radic Res 26: 381–398, [PUBMED], [INFOTRIEVE], [CSA]
- Robinson EE, Maxwell SRJ, Thorpe GHG (1997): An investigation of the antioxidant activity of black tea using enhanced chemiluminescence. Free Radic Res 26: 291–302, [PUBMED], [INFOTRIEVE], [CSA]
- Sasaki S, Ohta T, Decker EA (1996): Antioxidant activity of water-soluble fractions of salmon spermary tissue. J Agr Food Chem 44: 1682–1686, [CROSSREF], [CSA]
- Sawa T, Nakao M, Akaike T, Ono K, Maeda H (1999): Alkylperoxyl radical scavenging activity of various flavonoids and other phenolic compounds: Implications for the antitumor promoter effect of vegetables. J Agr Food Chem 47: 397–402, [CROSSREF], [CSA]
- Sharaf M, El-Ansari MA, Saleh NAM (1999): Flavone glycosides from Mentha longifolia.. Fitoterapia 70: 478–483, [CROSSREF], [CSA]
- Singleton VL, Orthofer RM, Ramuela-Raventos RM (1999): Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin Ciocalteu Reagent. Methods Enzymol 299: 152–178, [CSA]
- Squadriato GL, Peyor WA (1998): Oxidative chemistry of nitric oxide: The roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic Biol Med 25: 392–403, [CROSSREF], [CSA]
- Sun J, Chen Y, Li M, Ge Z (1998). Role of antioxidant enzymes on ionizing radiation resistance. Free Radic Biol Med 24: 586–593, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Takahashi O, Sakamoto Y, Hiraga K (1985): Lung hemorrhagic toxicity of butylated hydroxyanisole in the rat. Toxicol Lett 27: 15–25, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Tanizawa H, Ohkawa Y, Takino Y, Miyase T, Ueno A, Kageyama T, Hara S (1992): Studies on natural antioxidants in citrus species I. Determination of antioxidative activities of citrus fruits. Chem Pharm Bull 40: 1940–1942, [PUBMED], [INFOTRIEVE], [CSA]
- Terao J, Piskula M, Yao Q (1994): Protective effect of epicatechin, epicatechin gallate, and quercetin on lipid peroxidation in phospholipid bilayers. Arch Biochem Biophys 308: 278–284, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Ulubelen A, Topcu G, Kaya U (1994): Steroidal compounds from Teucrium chamaedrys. subsp. chamaedrys.. Phytochemistry 36: 171–173, [CROSSREF], [CSA]
- Ulubelen A, Topcu G, Sonmez U, Eris C, Ozgen U (1996): Norsesterterpenes and diterpenes from the aerial parts of Salvia limbata.. Phytochemistry 43: 431–434, [CROSSREF], [CSA]
- Valant-Vetschera KM, Roitman JN, Wollenweber E (2003): Chemodiversity of exudate flavonoids in some members of the Lamiaceae. Biochem Syst Ecol 31: 1279–1289, [CROSSREF], [CSA]
- Voirin B, Viricel MR, Favre-Bonvin J, Van den Broucke CO, Lemli J (1985): 5,6,4′-trihydroxy-7,3′-dimethoxyflavone and other metoxylated flavonoids isolated from Thymus satureioides.. Planta Med 6: 523–525, [CSA]
- Witschi HP (1981): Enhancement of tumor formation in mouse lung by dietary butylated hydroxytoluene. Toxicology 21: 95–104, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Yamamoto K, Tajima K, Mizutani T (1980): The acute toxicity of butylated hydroxytoluene and its metabolites in mice. Toxicol Lett 6: 173–175, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Yιldιrιm A, Oktay M, Bilaloğlu V (2001): The antioxidant activities of the leaves of Cydonia vulgaris. Turkish J Med Sci 31: 23–27, [CSA]
- Zgorka G, Glowniak K (2001): Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J Pharm Biomed Anal 26: 79–87, [PUBMED], [INFOTRIEVE], [CSA]