Abstract
Twenty-one furanocoumarins and related compounds were synthesized and screened as potential antitumor promoters by using the in vitro. short-term 12-O.-tetradecanoylphorbol-13-acetate (TPA)-induced Epstein-Barr virus early antigen (EBV-EA) activation assay. Compounds 3a and 5a showed the greatest potency (96% inhibition at 1000 mol ratio/TPA). Consequently, they were further tested in an in vivo. two-stage mouse skin carcinogenesis assay. Compounds 3a and 5a were slightly more potent than curcumin, a well-known antitumor promoter. These data suggested that furanocoumarins might be valuable antitumor promoters or chemopreventors.
Introduction
Furanocoumarins are an important bioactive compound class (Nair et al., Citation2002) and are reported to have anticoagulant, insecticidal, anthelmintic, hypnotic, antifungal, phytoalexin, and other physiological properties (Lee et al., Citation1998). These wide-ranging biological effects have stimulated interest in this compound type. As part of our interest in the development of agents for cancer chemoprevention, we synthesized 21 furanocoumarins and related compounds and screened them as potential antitumor promoters by examining their ability to inhibit Epstein-Barr virus early antigen (EBV-EA) activation induced by 12-O.-tetradecanoylphorbol-13-acetate (TPA) in Raji cells. We also conducted an in vivo. two-stage carcinogenesis test on mouse skin tumor promotion with compounds 3a and 5a, the most potent compounds in the EBV-EA assay. Herein, we will briefly report the synthesis and activities of the synthesized furanocoumarins and related compounds.
Materials and Methods
Chemistry
Compounds 2a–g, 3a–g, 4a–g, and 5a–g were prepared from variously substituted 4-hydroxy coumarins 1a–g according to well-reported routes (Rajitha et al., Citation1986; Majumdar & Bharracharyya, Citation1997), as shown in Scheme . Namely, the addition, followed by cyclization, of 4-hydroxy coumarins 1a–g with chloroacetaldehyde in the presence of K2CO3 in water at room temperature gave the corresponding 3-hydroxy-2,3-dihydrofuro[3,2-c][1]benzopyran-4-ones 2a–g in 60–80% yields (Majumdar & Bharracharyya, Citation1997). Dehydration of 2a–g with 2 N HCl at 50°C gave the desired furo[3,2-c]chromen-4-ones 3a–g in 90–95% yields (Majumdar & Bharracharyya, Citation1997). On the other hand, the treatment of 1a–g with chloroacetone in the presence of K2CO3 in acetone at 50°C produced the O-alkylated coumarins 4a–g in 30% yields. 3-Methylfuro[3,2-c]chromen-4-ones 5a–g were obtained in 90% yields by treating 4a–g with polyphosphoric acid (Rajitha et al., Citation1986).
Scheme 1 Synthetic routes to furanocoumarins and related compounds (i) chloroacetaldehyde, potassium carbonate; (ii) 2 N hydrochloric acid, water, 50°C; (iii) chloroacetone, potassium carbonate, acetone; (iv) polyphosphoric acid, 110°C.
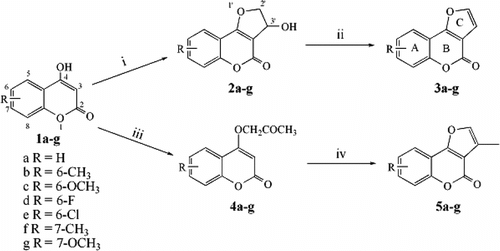
The target compounds were characterized on the basis of spectroscopic data, which were consistent with the expected products. The IR spectra of the products showed a strong peak around 1720 cm−1. This value is typical for the coumarin carbonyl group, indicating that the product has an angular skeleton (Nair et al., Citation2002). In the 1H NMR spectra, the aromatic proton peaks were seen between δ 7.31 and 7.86, and the protons on the dihydrofuran ring of compounds 2a–g displayed an expected ABX pattern [e.g., for 2f: δ 4.75 (1H, dd, J. = 2.7 Hz, 11.1 Hz, H-2′a), δ 4.85 (1H, dd, J. = 7.2 Hz, 11.1 Hz, H-2′b), δ 5.60 (1H, dd, J. = 2.7 Hz, 7.2 Hz, H-3′)].
In vitro. EBV-EA activation experiments
EBV-EA positive serum from a patient with nasopharyngeal carcinoma (NPC) was a gift from Professor H. Hattori (Department of Otorhinolaryngology, Kobe University). The EBV genome carrying lymphoblastoid cells (Raji cells derived from Burkitt lymphoma) were cultured in 10% fetal bovine serum (FBS) in RPMI-1640 medium (Sigma R8758, USA). Spontaneous activation of EBV-EA in our subline of Raji cells was less than 0.1%. The inhibition of EBV-EA activation was assayed using Raji cells (virus nonproducer type) as described below. The cells were incubated at 37°C for 48 h in 1 ml of medium containing n.-butyric acid (4 mM), TPA [32 pM = 20 ng in 2 µl dimethyl sulfoxide (DMSO)], and various amounts of the test compounds dissolved in 2 µl of DMSO. Smears were made from the cell suspension. The EBV-EA inducing cells were stained by means of an indirect immunofluorescence technique. In each assay, at least 500 cells were counted, and the number of stained cells (positive cells) was recorded. Triplicate assays were performed for each compound. The average EBV-EA induction of the test compound was expressed as a ratio relative to the control experiment (100%), which was carried out with n.-butyric acid (4 mM) plus TPA (32 pM). EBV-EA induction was ordinarily around 35%. The viability of treated Raji cells was assayed by the Trypan blue staining method. The cell viability of the TPA positive control was greater than 80%. Therefore, only these compounds that induced less than 80% (% of control) of the EBV-active cells (those with a cell viability of more than 60%) were considered able to inhibit the activation caused by promoter substances. Student's t.-test was used for all statistical analysis.
In vivo. two-stage carcinogenesis test on mouseskin papillomas
Specific pathogen-free female ICR mice (6 weeks old) were obtained from Japan SLC, Inc. (Hamamatsu, Japan) and were housed five per polycarbonate cage in a temperature-controlled room. All mice were fed oriental MF (Oriental Yeast Co., Tokyo, Japan) and water ad libitum. during the experiment. The animals were divided into four experimental groups of 15 mice each. The back of each mouse was shaved with surgical clippers, and the mice were treated topically with DMBA (100 µg, 390 nmol) in acetone (0.1 ml). One week after the initiation, papilloma formation was promoted twice a week by the application of TPA (1 µg, 1.7 nmol) in acetone (0.1 ml) on the skin. Group I received TPA treatment alone, group II received TPA and compound 5a (85 nmol), group III received TPA and compound 3a (85 nmol), and group IV received TPA and curcumin (85 nmol) in acetone (0.1 mL) 1 h before each TPA treatment. The incidence and numbers of papillomas were detected weekly for 20 weeks. Student's t.-test was used for all statistical analysis.
Results and Discussion
The primary screening was carried out using a short-term in vitro. synergistic assay on EBV-EA activation induced by TPA (Henle & Henle, Citation1966; Takasaki et al., Citation1990). lists inhibitory effects of tested compounds and the associated viability of Raji cells. Curcumin, a natural folk medicine and widely studied anti-inflammatory compound, apoptosis inducer, and chemopreventive agent, was used as the positive control (Duvoix et al., Citation2005). In this assay, all compounds showed inhibitory effects on EBV-EA activation without high cytotoxicity on Raji cells. As shown in , compounds 3a, 5a, and 5f showed significant inhibitory effects (more than 95% inhibition of activation at 1000 mol ratio/TPA, 60–65% inhibition at 500 mol ratio/TPA, and 30–35% inhibition at 100 mol ratio/TPA). Because inhibitory effects on EBV-EA activation induced by the tumor promoter TPA generally correlate well with inhibitory effects on tumor promotion in vivo. (Konoshima et al., Citation1994; Ishida et al., Citation2000; Sakurai et al., Citation2003), the in vitro. results strongly suggested that these furanocoumarins might also be valuable antitumor promoters.
Table 1 Relative ratioFootnotea. of Epstein-Barr virus early antigen (EBV-EA) activation with respect to positive control (100%) in presence of furanocoumarin and analogues.
The effects of two of the most active compounds, 3a and 5a, on the two-stage carcinogenesis test of mouse skin papillomas were investigated using DMBA as an initiator and TPA as a promoter (Tokuda et al., Citation1986). The incidence (%) of papilloma-bearing mice and the average numbers of papillomas per mouse are presented in A and B, respectively.
Figure 1 Inhibition of TPA-induced tumor promotion by multiple applications of furanocoumarins 5a and 3a. All mice were carcinogenically initiated with DMBA and promoted with 1.7 nmol of TPA given twice weekly starting 1 week after initiation. (A) Percentage of mice bearing papillomas; (B) average number of papillomas per mouse. At 20 weeks of promotion, groups II, III, and IV were different from group I (p < 0.05) in numbers of papillomas per mouse.
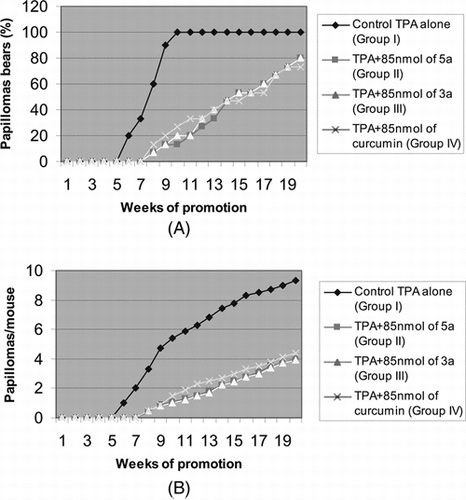
As shown in A, 100% of the mice in the control group (group I, untreated) bore papillomas as early as 10 weeks of promotion. Furthermore, averages of 5.4 and 9.3 papillomas were formed per mouse at 10 and 20 weeks of promotion, respectively, as shown in B. On the other hand, in all treated groups, the formation of papillomas in mouse skin was delayed, and the mean numbers of papillomas per mouse were reduced. Compared with untreated group I, the numbers of papilloma-bearing mice were reduced to 13.3% and 80% at 10 and 20 weeks, respectively, in group II treated with 5a and to 20% and 80% in group III treated with 3a (A). Also, only 1.1 and 1 papillomas were found per mouse at 10 weeks and 4.0 and 3.9 papillomas per mouse at 20 weeks of promotion in groups II and III, respectively (B). In group IV treated with curcumin, the numbers of papilloma-bearing mice were 35% and 93% of the positive control at 10 and 20 weeks, respectively (A), with 1.6 and 4.9 papillomas per mouse at 10 and 20 weeks of promotion, respectively (B) (Balasubramanian et al., Citation1997). Thus, compounds 3a and 5a showed better in vitro. and in vivo. activity than curcumin, which is a known antitumor promoter.
From the results obtained in the current study, the following comments can be made. The most potent compounds were 3a, 5a, 5f, 3d, 2a, 2d, 4b, and 5b. Substituents on the A ring affected the potency. With the exception of 4a, the unsubstituted analogs (2a, 3a, and 5a) were among the most active in each series. The 6-fluorinated 2d and 3d showed potency comparable with the corresponding unsubstituted compounds, and compounds with fluorine were more active than those with chlorine at the 6-position in three series (2d/e, 3d/e, and 5d/e). Notably, methylation at either the 6- or 7-position greatly increased the potency relative to methoxylation in two series, (4b/c, 5b/c and 4f/g, 5f/g), although it had less or no effect in the other two series. At the highest dose tested, all seven furanocoumarins 3a–g showed ≥ 90% inhibition in vitro. () and were more potent than the O-alkylated coumarins 4a–g, with the notable exception of 4b.
Conclusions
In summary, furanocoumarin 3a and 3′-methylfuranocoumarin 5a showed both high in vitro. and in vivo. activity in this investigation. Further study is needed on the structure-activity relationships of furanocoumarins and analogues; however, 6-fluorination or -methylation and 7-methylation could positively affect the potency of certain compound classes. This investigation showed that furanocoumarins are likely valuable as antitumor promoters or as lead compounds for new chemopreventive drug development.
Acknowledgments
This investigation was supported in part by a grant from the National Cancer Institute (CA 17625) awarded to K.H. Lee. This study was also supported in part by a grant from the Ministry of Education, Sciences, Sports and Culture, and Ministry of Health and Welfare, Japan (Kyoto).
References
- Balasubramanian V, Kapadia GJ, Tokuda H, Takayyasu J, Konoshima T, Takasaki M, Nishino H (1997): Abstract of the 38th Annual Meeting of the American Society of Pharmacognosy, Iowa City, IA, USA, July 27, poster 82.
- Duvoix A, Blasius R, Delhalle X, Schnekenburger M, Morceau F, Henry E, Dicato M, Diederich M (2005): Chemopreventive and therapeutic effects of curcumin. Cancer Lett 223: 181–190, [PUBMED], [INFOTRIEVE], [CSA]
- Henle G, Henle W (1966): Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol 91: 1248–1256, [PUBMED], [INFOTRIEVE], [CSA]
- Ishida J, Kozuka M, Wang HK, Konoshima T, Tokuda H, Okuda M, Mou XY, Nishino H, Sakurai N, Lee KH, Nagai M (2000): Antitumor-promoting effects of cyclic diarylheptanoids on Epstein-Barr virus activation and two-stage mouse skin carcinogenesis. Cancer Lett 159: 135–140, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Konoshima T, Takasaki M, Tatsumoto T, Kozuka M, Kasai R, Tanaka O, Nie RL, Tokuda H, Nishino H, Iwashima A (1994): Inhibitory effects of cucurbitane triterpenoids on Epstein-Barr virus activation and two-stage carcinogenesis of skin tumors. Biol Pharm Bull 17: 668–671, [PUBMED], [INFOTRIEVE], [CSA]
- Lee YR, Kim BS, Wang HC (1998): Silver(I)/celite promoted oxidative cycloaddition of 4-hydroxycoumarins to olefins. A facile synthesis of dihydrofurocoumarins and furocoumarins. Tetrahedron 54: 12215–12222, [CROSSREF], [CSA]
- Majumdar KC, Bhattacharyya T (1997): Regioselective synthesis of furo[3,2-c][1]benzopyran-4-one and furo[3,2-c]quinolin-4-one. J Chem Res 244–245, [CROSSREF], [CSA]
- Nair V, Menon RS, Vinod AU, Viji S (2002): A facile three-component reaction involving [4 + 1] cycloaddition leading to furan annulated heterocycles. Tetrahedron Lett 43: 2293–2295, [CROSSREF], [CSA]
- Rajitha B, Geetanjali Y, Somayajulu VV (1986): Synthesis of coumestrol analogs as possible antifertility agents. Indian J Chem 25B: 872–873, [CSA]
- Sakurai N, Kozuka M, Tokuda H, Nobukuni Y, Takayasu J, Nishino H, Kusano A, Kusano G, Nagai M, Sakurai Y, Lee KH (2003): Antitumor agents 220. Antitumor-promoting effects of cimigenol and related compounds on Epstein-Barr virus activation and two-stage mouse skin carcinogenesis. Bioorg Med Chem 11: 1137–1140, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Takasaki M, Konoshima T, Fujitani K, Yoshida S, Nishimura H, Tokuda H, Nishino H, Iwashima A, Kozuka M (1990): Inhibitors of skin-tumor promotion. VIII. Inhibitory effects of euglobals and their related compounds on Epstein-Barr virus activation. (1). Chem Pharm Bull 38: 2737–2739, [PUBMED], [INFOTRIEVE], [CSA]
- Tokuda H, Ohigashi H, Koshimizu K, Ito Y (1986): Inhibitory effects of ursolic and oleanolic acid on skin tumor promotion by 12-O.-tetradecanoylphorbol-13-acetate. Cancer Lett 33: 279–285, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
