Abstract
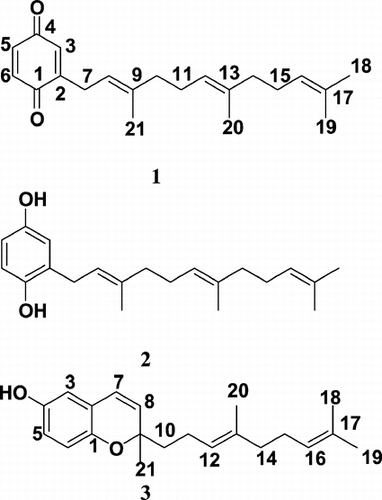
The SRB cytotoxicity assay was used to screen plant extracts, in a collaborative multinational OAS project involving Argentina, Bolivia, Colombia, Costa Rica, Guatemala, Nicaragua and Panama, against breast (MCF-7), lung (H-460), and central nervous system (SF-268) human cancer cell lines. Out of 310 species tested, 23 (7.4%) plants showed cytotoxic activity at GI50 values ≤10 µg/ml. The most active plants were Thevetia ahouai., Physalis viscosa., Piper jacquemontianum., Piper barbatum., Senna occidentalis., Tovomita longifolia., and Lippia cardiostegia.. Blepharocalyx salicifolius. and Senna occidentalis. were selectively active against one cell line, SF-268 or MCF-7, respectively. Within the framework of this project, 14 compounds have been isolated, 5 new (4 benzophenones, coumarin) and 9 known to the literature. But only the bioassay-guided fractionation of the active extract of Piper barbatum. leaves, which led to the isolation of three known compounds: (2′E., 6′E.)-2-farnesyl-1,4-benzoquinone (1), (2′E., 6′E.)-2-farnesylhydroquinone (2), and dictyochromenol (3), is reported here. The chemical structures of 1 and 2 were determined by spectral means (1D, 2D NMR, MS) and chemical data. Among these three, (2′E., 6′E.)-2-farnesyl-1,4-benzoquinone was the most active (MCF-7 GI50 = 1.8 µg/ml; H-460 GI50 = 4.8 µg/ml; SF-268 GI50 = 3.5 µg/ml).
Introduction
Cancer is a public health problem worldwide. According to WHO, 20 million people in the world suffer from cancer, a figure projected to rise to 30 million within 20 years (WHO, Citation2004). Cancer causes 7.1 million deaths annually (12.6% of the global total). In Latin America, cancer has been ranked as one of the first three leading causes of death (PAHO, Citation2002).
The plant kingdom has been a valuable source of several clinically useful anticancer agents such as vinblastine, vincristine, the camptothecin derivatives, topotecan and irinotecan, etoposide, derived from epipodophyllotoxin, and paclitaxel (Taxol). Moreover, a number of promising new anticancer agents are in clinical development at the moment, including flavopiridol [synthetic analogue of rohitukine isolated from Dysoxylum binectariferum. Hook. F. (Meliaceae)] and combrestatin A4 phosphate [natural product isolated from Combretum caffrum. (Eckl. & Zeyh.) Kuntze (Combretaceae)] (Cragg & Newman, Citation2005).
An analysis of the distribution of higher plants by continents indicates that Latin America possesses a higher number of vascular plants (85,000 plants) than tropical and subtropical Asia (50,000 plants) (World Conservation Monitoring Center, Citation1992). Although it was believed in the past that the forests from Southeast Asia were more diverse, there is recent evidence indicating that neotropical forests located in Latin America possess higher diversity of plants in the world (Berry, Citation2002).
In order to explore rationally the potential of the plant diversity of the region, a multinational OAS project, comprising the multidisciplinary collaborative participation of research centers in Argentina, Bolivia, Colombia, Costa Rica, Guatemala, Nicaragua, and Panama, was carried out during the period 2001–2004. This project aimed at screening organic plant extracts for antifungal, anti-trypanosomal, anti-leishmanial, antimalarial, and cytotoxic activities and subsequently to isolate and characterize bioactive molecules.
For this, plants from a database of ethnomedical uses of Latin American plants, PLANMEDIA (CIFLORPAN, Citation2004), were selected according to the amount of biological and chemical information available in the literature for cytotoxicity screening in order to identify potential anticancer plants of the region. We report here screening of 452 plant extracts for cytotoxicity using the sulforhodamine assay and the isolation of compounds from the most active plant Piper barbatum.. Results of antiprotozoal screening will be reported later.
Materials and Methods
Plant material
Plants were collected mainly from tropical forests in the seven countries (Argentina, Bolivia, Colombia, Costa Rica, Guatemala, Nicaragua, and Panama). Their taxonomic identity was established by the botanists Martha Gattuso, M. Frecentese, Elisa Petenatti (Argentina); Rosy de Michel, Genevieve Bourdy (Bolivia); Ricardo Callejas, Edgar Linares, José Luis Fernández, Zaleth Cordero, Santiago Díaz, N.R. Salinas (Colombia); Luis Guillermo Acosta, Luis D. Vargas, Alexánder Rodríguez (Costa Rica); Mario Veliz, Elfriede de Pöll, Juan José Castillo (Guatemala); Ricardo Rueda, Dania Paguaga, Hilario Mendoza, Nelson Toval y Miguel Garmendia (Nicaragua); Mireya Correa (Panama); and the voucher specimens are deposited at the corresponding National Herbaria in each country: Herbarium of the National University of San Luis (UNSL), Argentina, and Herbarium of the National University of Rosario (UNR), Argentina; National Herbarium of Bolivia (HLP), Bolivia; National Colombian Herbarium (COL), Colombia; Herbarium of INBio, Costa Rica; Herbarium of Farmaya, Guatemala; Universidad Nacional Autónoma de Nicaragua-León (Herbario UNAN-León; HULE), Nicaragua; and Herbarium of the University of Panama (PMA), Panama ().
Table 1 Cytotoxic extracts of selected plants.
Selection of plants
A random list of plants from the ethnomedical database PLANMEDIA (CIFLORPAN, Citation2004) was submitted to a NAPRALERT search for biological and chemical information. From that list, 314 plant species were prioritized according to the amount of information found in the literature.
Preparation of extracts
In general, plant material was macerated with 80% ethanol (24 h) for extraction (3-times). The plant extracts were filtered and concentrated in vacuo. at <40°C in a rotary evaporator and stored at −80°C until further use.
Cytotoxicity bioassays
The cytotoxic activity against breast (MCF-7), lung (H-460), and central nervous system (SF-268) human cancer cell lines was determined according to the method of Monks et al. (Citation1991Citation1997). The cell lines were obtained from the U.S. National Cancer Institute. Adriamycin was used as standard.
Isolation of cytotoxic compounds
The leaves of P. barbatum. (853 g) were extracted according to the procedure described above, resulting in 130 g of crude extract. The EtOH extract was then partitioned successively with CHCl3, hexane, MeOH 90%, EtOAc, and BuOH. The cytotoxic activity was retained in the hexane (115 g) partition. The hexane partition was further chromatographed over silica gel with hexane:EtOAc (0 → 100%) mixtures in order of increasing polarity, followed by EtOAc:MeOH (0 → 100%) mixtures to give 126 fractions (A–V) of 500 ml each.
The active fraction L (68.1 g) was submitted to silica gel chromatography and eluated with gradients of hexane:EtOAc (0 → 100%) and EtOAc:MeOH (0 → 100%) to give 39 fractions (L-1 to L-10) of 250 ml each.
The major component of fraction L-4 (419.5 mg) was subjected to a silica gel column chromatography with solvent gradients of toluene-EtOAc (0 → 100%), followed by further purification using Sephadex column in CHCl3 100% to give compound 1 (52 mg).
The active fraction L-5 (2.2 g) was further chromatographed on silica gel 60 (0.063–0.200 mm) with a solvent gradient of hexane-CH2Cl2 (0 → 100%). Further purification of two main components by Sephadex LH-20 in CHCl3 100% was carried out to afford two main fractions of 10 ml each (L-5a and L-5b). Crystallization of fraction L-5a with CHCl3 100% afforded compound 2 (120 mg). Fraction L-5b furnished compound 3 (214.7 mg).
Results and Discussion
Ethnomedical database
This collaborative multinational OAS project aimed at discovering novel bioactive molecules during 2001–2004 has firstly resulted in the generation of an ethnomedical database PLANMEDIA with a total of 4129 entries corresponding to 1152 species (167 families and 692 genera). The establishment of this database was intended to keep vast knowledge in traditional medicine in Latin America mentioned by Pedersen and Baruffati (Citation1985). This is supported by the fact that Latin America has a high percentage of native indigenous people who depend upon traditional medicine for treating their ailments (Gupta, Citation2005).
Cytotoxicity of extracts
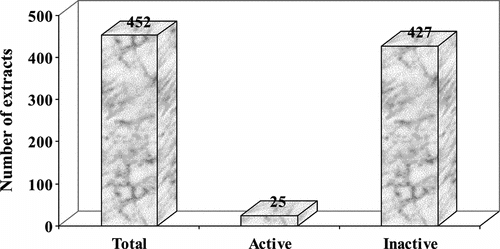
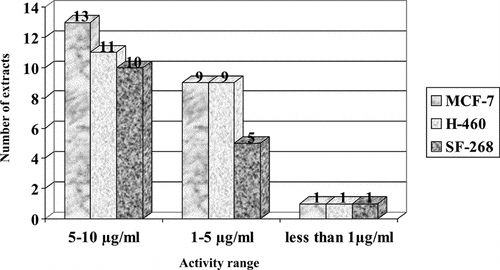
In addition, from 311 plant species collected, 452 extracts were prepared and tested in the cytotoxicity assay. Out of 452, 81 extracts were tested in five concentrations (100, 31.6, 10.0, 3.17, 1.0 µg/ml) to determine the GI50 values (Tables and ). Forty-nine percent of plants tested at this stage belong to the major families such as Asteraceace (18%), Piperaceae (16%), Rubiaceae (5%), Solanaceae (5%), and Fabaceae (5%). The extracts with GI50 values ≤10 µg/ml were considered as active. Twenty-five (5.5%) extracts (; ), representing 23 species, 21 genera, 18 families, showed relevant cytotoxic activity. The cytotoxic activities of extracts distributed in three ranges of concentration (5–10 µg/ml, 1–5 µg/ml, ≤1 µg/ml) are shown in . The more active cytotoxic extracts in the biological screening are summarized in .
Table 2 Inactive plant extracts.
The most active plant against the three cancer cell lines was Thevetia ahouai. with the following GI50 values [MCF-7 (µg/ml): 0.47; H-460 (µg/ml): 0.29; SF-268 (µg/ml): 0.52]. Two plants showed selectivity against one specific cancer cell line: Blepharocalyx salicifolius. IC50 (µg/ml) 9.0 MCF-7 and Senna occidentalis. IC50 (µg/ml) 3.9 SF-268.
Thevetia ahouai. and Annona muricata. reported ethnomedical use against cancer in several Latin American countries (Gupta et al., Citation1986; García Barriga, Citation1992). The ethnomedical use of these two plants may be corroborated with their cytotoxic activity found in our biological screening (). This is further supported by the presence of known cytotoxic compounds in these plants, as follows: (a) three cardenolide glycosides, neriifolin, 3′-O.-methylevomonoside and 2′-acetyl-neriifolin, have been isolated from T. ahouai.. These compounds exhibited a distinctive pattern of differential cytotoxicity in the National Cancer Institute's human disease–oriented 60-cell-line tumor screening panel (Decosterd et al., Citation1994); (b) A. muricata., like all plants of the family Annonaceae, contains acetogenins. There are several reports on cytotoxic mono and bis-tetrahydrofuran acetogenins isolated from A. muricata. (Rieser et al., Citation1996; Kim et al., Citation1998; Chang & Wu, Citation2001; Liaw et al., Citation2002; Chang et al., Citation2003; Suksamrarn et al., Citation2004).
Flavanones and flavones found in the genus Chromolaena. showed moderate cytotoxicity against human small-cell lung cancer (NCl-H187) cells (Suksamrarn et al., Citation2004). This evidence in the literature and cytotoxic activity [IC50 values MCF-7 (µg/ml): 7.5; H-460 (µg/ml): 7.1; SF-268 (µg/ml): 7.5] of Chromolaena leivensis. in the current study may corroborate the ethnomedical use of species of genus for the treatment of tumors (Gupta, Citation1995).
Cytotoxic activities of Enterolobium contortisiliquum. against BAC1.2F5 mouse macrophages, EL-4 mouse lymphoma cells, and L-929 mouse fibroblasts have been reported due to the presence of bisdemosidic saponin (Mimaki et al., Citation2003Citation2004). This type of saponin could be responsible for the cytotoxicity shown against H-460 and SF-268 cell lines in the current study.
Physalins (13,14-seco.-16,24-cyclosteroids) from Physalis. species have shown cytotoxic activity against HeLa cells (Makino et al., Citation1994Citation2003; Kawai et al., Citation2002). A cytotoxic flavonoid glycoside isolated from Physalis angulata. L. showed remarkable cytotoxicity in vitro. against murine leukemia cell line P-388, epidermoid carcinoma of the nasopharynx KB-16 cells, and lung adenocarcinoma A-549 (Ismail & Alam, Citation2001). Physalis viscosa., as member of the cytotoxic Physalis. genus, displayed cytotoxicity against the three cell lines in the current work.
In the genus Piper., pyridone alkaloids exhibited cytotoxicity against P-388, HT-29, or A549 and KB cell lines in vitro. (Duh et al., Citation1990; Chen et al., Citation2003), and amides exerted cytotoxicity (cell survival <15%) against CCRF-CEM, HL-60, PC-3, and HA22T cell lines (Chen et al., Citation2002). As part of this genus Piper., P. jacquemontianum., P. barbatum., and P. glabratum. showed significant cytotoxicity against the three cell lines.
Two new [(E.)-3-(2-hydroxy-7-methyl-3-methyleneoct-6-enyl)-2,4,6-trihydroxybenzo phenone; 8-benzoyl-2-(4-methylpenten-3-yl)chromane-3,5,7-triol] and one known (4-geranyloxy-2,6,-dihydroxybenzophenone) benzophenones isolated from Tovomita longifolia. presented cytotoxicity against the three cell lines in this project (Pecchio et al., Citation2006). Previously, two known xanthones, trapezifolixanthone and manglexanthone, were isolated as cytotoxic constituents from the CHCl3 extract of the roots of other Tovomita. species by bioassay-guided fractionation using the KB cell line (Seo et al., Citation1999).
As part of this project, three cytotoxic cucurbitacins (23,24-dihydro cucurbitacin F, 23,24-dihydro-25-acetylcucurbitacin F and 2-O-β-D-glucopyranosyl-23,24-dihydrocucurbitacin F) were found in fruits of Courtarea hexandra. (Olmedo et al., Citation2005).
Currently, no data are available regarding the in vitro. cytotoxic activity for the species or genera of Blepharocalyx salicifolius., Cordia cylindrostachya., Coutarea hexandra., Hypericum uliginosum., Jacquinia nervosa., Larrea cuneifolia., Lippia cardiostegia., Melampodium divaricatum., Monochaetum myrtoideum., Myrica pubescens., Senna occidentalis., Trichospermum galeottii., and Unonopsis theobromifolia., and thus are interesting for further studies.
Identification of isolated compounds
Compound 1 was identified as (2′E., 6′E.)-2-farnesyl-1,4-benzoquinone, 2 as (2′E., 6′E.)-2-farnesylhydroquinone, and 3 as dictyochromenol by comparison of their spectral data () with those reported in the literature (Muanza-Nkongolo et al., Citation1984; Peña et al., Citation2000). All three compounds are known compounds. Compounds 1 and 2 have been previously found in fruits of Piper barbatum. C. DC. (synomym: Piper bogotense. C. DC.) (Peña et al., Citation2000) but not in the leaves, which was the part studied in this project. Compound 2 was isolated for the first time from the brown seaweed Dictyopteris undulata. Holmes (Ochi et al., Citation1979) and subsequently from Wigandia kunthii. Choisy (Hydrophyllaceae) (Gomez, Citation1980). Compound 3 has been only reported in the brown alga Dictyopteris undulata. (Muanza-Nkongolo et al., Citation1984). This is the first report of its presence in higher plants.
Table 3 1H and 13C NMR spectroscopic data for compounds 1, 2, 3.
Within the framework of this study, 14 compounds have been isolated, 5 new (4 benzophenones, 1 coumarin) and 9 known to the literature.
Cytotoxicity of isolated compounds
Compound 1 showed the highest cytotoxic activity (GI50 1.8 µg/ml) against the MCF-7 cancer cell line. There is no significant difference for the cytotoxic activity against H-460 among the three compounds (). Furthermore, compound 2 displayed more activity (GI50 1.6 µg/ml) against SF-268 than compounds 1 and 3, which showed similar activities against this cancer cell line. The isolated compounds are shown in .
Although compounds 1 and 2 were more active against one cell line (MCF-7 or SF-268, respectively) than the others, there is not enough evidence of selectivity from these data. Further investigation of their cytotoxic activity against other cancer cell lines needs to be carried out.
This is the first report of the in vitro. cytotoxic activities against the human cancer cell lines MCF-7, H-460 and SF-268 of (2′E.,6′E.)-2-farnesyl-1,4-benzoquinone (1), (2′E.,6′E.)-2-farnesylhydroquinone (2), and dictyochromenol (3).
Conclusions
This study has identified a number of plant extracts () that have shown in vitro. cytotoxic activities, based on the selection of plants present in the database PLANMEDIA. The results of the cytotoxicity screening revealed that 3 out of the 23 active plants belong to the genus Piper.. In the Thevetia ahouai. and Annona muricata. species examined, their ethnomedical uses were confirmed with the cytotoxic activity presented in the screening.
Acknowledgments
This work was supported by the Organization of American States (OAS) through the Multinational project “Aprovechamiento de la Flora Regional como Fuente de Moléculas Antifúngicas, Antiparasitarias y Anticancer” (SEDI/AICD/106/01) y Convenio Andrés Bello (SECAB).
References
- Berry PE (2002): Diversidad y endemismo en los bosques neotropicales de bajura. In: Guariguata MR, Catan GH, eds., Ecología y Conservación de Bosques. Cartago, Costa Rica, Libro Universitario Regional (EULAC-GTZ), pp. 83–96.
- Chang FR, Wu YC (2001): Novel cytotoxic annonaceous acetogenins from Annona muricata.. J Nat Prod 64: 925–931, [PUBMED], [INFOTRIEVE], [CSA]
- Chang F, Liaw C, Lin C, Chou C, Chiu HF, Wu YC (2003): New adjacent bis.-tetrahydrofuran annonaceous acetogenins from Annona muricata.. Planta Med 69: 241–246, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Chen JJ, Huang YC, Chen YCh, Huang YT, Wang SW, Peng CY, Teng CM, Chen IS (2002): Cytotoxic amides from Piper sintenense.. Planta Med 68: 980–985, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Chen JJ, Duh CY, Huang HY, Chen IS (2003): Cytotoxic constituents of Piper sintenensis.. Helv Chim Acta 86: 2058–2064, [CROSSREF], [CSA]
- CIFLORPAN (2004): Base de Datos de Usos Ethnomédicos de Plantas Panameñas y Centroamericanas (PLANMEDIA). Project SEDI/AICD/106/01-04. Washington, DC, Organization of American States.
- Cragg GM, Newman DJ (2005): Plants as a source of anti-cancer agents. J Ethnopharmacol 100: 72–79, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Decosterd L, Gustafson KR, Cardellina JH II, Cragg GM, Boyd MR (1994): The differential cytotoxicity of cardenolides from Thevetia ahouia.. Phytotherapy Res 8: 74–77, [CSA]
- Duh CY, Wu YC, Wang SK (1990): Cytotoxic pyridone alkaloids from the leaves of Piper aborescens.. J Nat Prod 53: 1575–1577, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- García Barriga H (1992): Flora Medicinal de Colombia, Vol. II. Bogotá, Tercer Mundo, p. 456.
- Gómez F, Quijano L, Calderón JS, Ríos T (1980): Terpenoids isolated from Wigandia kunthii.. Phytochemistry 19: 2202–2203, [CROSSREF], [CSA]
- Gupta MP, Solís P, Soto A, Cedeño J, Correa M (1986): Observaciones etnofarmacognósticas sobre plantas medicinales en Panamá. Parte II. Scientia (Panama) 1: 43–57, [CSA]
- Gupta M (1995): 270 Plantas medicinales Iberoamericanas. CYTED. Convenio Andrés Bello. Bogotá, CYTED, p. 104.
- Gupta MP (2005): Regional overview: Region of the Americas. In: Bodeker G, Ong CK, Grundy C, Burford G, Shein K, eds., WHO Global Atlas of Traditional, Complementary and Alternative Medicine. Text volume. Kobe, Japan, World Health Organization Centre for Health Development, p. 41.
- Ismail N, Alam M (2001): A novel cytotoxic flavonoid glycoside from Physalis angulata.. Fitoterapia 72: 676–679, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Kawai M, Makino B, Yamamura H, Araki S, Butsugan Y, Ohya J (2002): Cytotoxic activity of physalins and related compounds against HeLa cells. Pharmazie 57: 348–350, [PUBMED], [INFOTRIEVE], [CSA]
- Kim GS, Lu Z, Alali F, Rogers LL, Wu FE, Sastrodihardjo S, McLaughlin JL (1998): Muricoreacin and murihexocin C, monotetrahydrofuran acetogenins, from the leaves of Annona muricata.. Phytochemistry 49: 565–571, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Liaw CC, Chang FR, Lin CY, Chou CJ, Chiu HF, Wu MJ, Wu YC (2002): New cytotoxic monotetrahydrofuran annonaceous acetogenins from Annona muricata.. J Nat Prod 65: 470–475, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Makino B, Kawai M, Yamamoto T, Furuta T, Iwata Y, Yamamura H, Butsugan Y, Ogawa K, Hayashi M, Ohya J (1994): Interrelation, structural modifications, and antitumor activity of physalins, highly-oxygenated steroids isolated from Physalis. plants. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 36: 1–8, [CSA]
- Makino B, Ohya J, Yamamura H, Araki S, Butsugan Y, Kawai M (2003): Cytotoxic activity of physalins possessing modified skeletal structures against HeLa cells. Pharmazie 58: 70–71, [PUBMED], [INFOTRIEVE], [CSA]
- Mimaki Y, Harada H, Sakuma C, Haraguchi M, Yui S, Kudo T, Yamazaki M, Sashida Y (2003): Enterolosaponins A and B, novel triterpene bisdesmosides from Enterolobium contortisiliquum., and evaluation for their macrophage-oriented cytotoxic activity. Bioorg Med Chem Lett 13: 623–627, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Mimaki Y, Harada H, Sakumna C, Haraguchi I, Yui S, Kudo T, Yamazaki M, Sashida Y (2004): Contortisiliosides A-G: Isolation of seven new triterpene bisdesmosides from Enterolobium contortisiliquum. and their cytotoxic activity. Helv Chim Acta 87: 851–865, [CROSSREF], [CSA]
- Monks A, Scudeiro D, Skehan P, Shoemaker R, Pauli K, Vistica D, Hosse C, Langley J, Cronise P, Vaifgro-Wolff A, Gray-Goodrich M, Campbell H, Mayo J, Boyd M (1991): Feasibility of high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83: 757–766, [PUBMED], [INFOTRIEVE], [CSA]
- Monks A, Scudiero DA, Jonson GS, Pauli KD, Sausville EA (1997): The NCI-anticancer drug screen: A smart screen to identify effectors of novel targets. Anti-cancer Drug Des 12: 533–541, [CSA]
- Muanza-Nkongolo D, Takenori K, Ishitsuka M, Iwashita T, Kakisawa H (1984): A piscicidal chromanol and chromenol from the brown alga Dictyopteris undulata.. Heterocycles 22: 2301–2307, [CSA]
- Ochi M, Kotsuki H, Inoue S, Taniguchi M, Tokoroyama T (1979): Isolation of 2-(3,7,11-trimethyl-2,6,10-dodecatrienyl)hydroquinone from the brown seaweed Dictyopteris undulata.. Chem Lett 7: 831–832, [CSA]
- Olmedo D, Rodríguez N, Vásquez Y, Espinosa A, Solís PN, Gupta MP (2006): New coumarin from the fruits of Coutarea hexandra.. Jornadas Iberoamericanas de Resonancia Magnética Nuclear: Avances en Resonancia Magnética Nuclear e Interacciones Moleculares. Agencia Española de Coperación Internacional (AECI) –CYTED. October 3–7. Santa Cruz de la Sierra, Bolivia.
- PAHO (2002): Health in the Americas, Vol. I. Edition 2002. Washington, DC, Pan American Health Organization, p. 15.
- Pecchio M, Solís P, López-Pérez JL, Vázquez Y, Rodríguez N, Olmedo D, Correa M, San Feliciano A, Gupta MP (2006): Cytotoxic and antimicrobial benzophenone derivatives from the leaves of Tovomita longifolia.. J Nat Prod 69: in press, , [CSA]
- Pedersen D, Baruffati V (1985): Health and traditional medicine cultures in Latin America and the Caribbean. So Sci Med 21: 5–12, [CROSSREF], [CSA]
- Peña LA, Avella E, Puentes de Diaz AM (2000): Benzoquinona e hidroquinona preniladas y otros constituyentes aislados de Piper bogotense. C. DC. Rev Col Quím 29: 25–37, [CSA]
- Rieser MJ, Gu ZM, Fang XP, Zeng L, Wood KV, McLaughlin JL (1996): Five novel mono-tetrahydrofuran ring acetogenins from the seeds of Annona muricata.. J Nat Prod 59: 100–108, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Seo EK, Wall ME, Wani MC, Navarro H, Mukherjee R, Farnsworth NR, Kinghorn AD (1999): Cytotoxic constituents from the roots of Tovomita brevistaminea.. Phytochemistry 52: 669–674, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Suksamrarn A, Chotipong A, Suavansri T, Boongird S, Timsuksai P, Vimuttipong S, Chuaynugul A (2004): Antimycobacterial activity and cytotoxicity of flavonoids from the flowers of Chromolaena odorata.. Arch Pharmacal Res 27: 507–511, [CSA]
- World Conservation Monitoring Center (1992): Global Diversity. Status of the Earth's Living Resources. London, Chapman & Hall.
- WHO (2004): The World Health Report 2004. Geneva, WHO.