Abstract
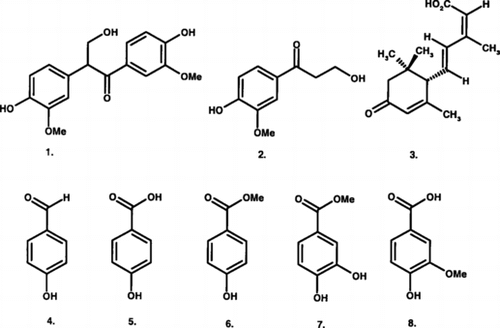
1,2-bis.-(4-Hydroxy-3-methoxyphenyl)-3-hydroxypropan-1-one (evafolin B, 1), 1-(4-hydroxy-3-methoxyphenyl)-3-hydroxypropan-1-one (2), were isolated for the first time from the genus Schisandra., along with abscisic acid (3), 4-hydroxybenzaldehyde (4), 4-hydroxybenzoic acid (5), methyl 4-hydroxybenzoate (6), methyl 3,4-dihydroxybenzoate (7), and vanillic acid (8). All the compounds were evaluated for their antitumor, antiproliferative, and antioxidant activities. Only compound 7 exhibited moderate activity against three human cancer cell lines and human lymphocytic proliferation, as well as strong inhibitory activity for DPPH free radicals, only slightly less than ascorbic acid.
Introduction
Plants of the genus Schisandra. belong to the economically and medicinally important family Schisandraceae. This genus comprises about 39 species, which are mainly distributed in East Asia with China as the center of diversity. Much attention has been focused on Schisandra chinensis. due to its medicinal properties including anticancer, tonic, and antiaging (Kuo et al., Citation1997). Though several dibenzocyclooctadiene lignans from S. chinensis. have been found to have some beneficial effects including antihepatitis activity and antioxidant activity and to be involved in nervous system regulation (Hancke et al., Citation1999), lignans with interesting biological activity have been recently isolated from other species of Schisandra. (Li et al., Citation2004b; Chen et al., Citation2005). Consequently, lignans from Schisandra. seem to be a potential source of new synthetic drugs, as in the case of BDD (Jiaxiang et al., Citation1993). For example, halogenated gomisin J derivatives have been shown to possess anti-human immunodeficiency virus (HIV) activity by inhibiting the activity of the enzyme reverse transcriptase as well as expressing cytoprotective activity in HIV-1–infected H9 cells (Fujihashi et al., Citation1995). Besides lignans, several triterpenoids have been found to be important constituents of many species of Schisandra. (Li et al., Citation2003Citation2004a). While nigranoic acid, isolated from S. sphaerandra., was found to be the HIV-1 transcriptase inhibitor (Sun et al., Citation1996), manwuweizic acid from S. propinqua. showed a significant cytotoxic effect against human decidual cells and rat luteal cell in vitro. (Chen et al., Citation2001).
In our continuing search for new biologically active secondary metabolites from the medicinal plants in northern Thailand aligned with the medicinal interest of the plants of the genus Schisandra., we have investigated the constituents of Schisandra verruculosa., the only species found in Thailand. Besides two phenylpropanoid derivatives (1, 2), abscisic acid (3), 4-hydroxybenzaldehyde (4), and four benzoic acid derivatives (5–8) have been isolated from its stem wood. The structure of the compounds was established by spectral analysis (1H, 13C, COSY, HSQC, HMBC, and NOESY) as well as HRMS. All the isolated compounds were evaluated for their in vitro. effect on growth of three human cancer cell lines: MCF-7 (breast), NCI-H460 (lung), SF-286 (CNS), and on the mitogenic response of human lymphocytes to phytohemagglutinin as well as for their scavenging capacity for DPPH free radical.
Materials and Methods
General experimental procedure
1H and 13C NMR were recorded at ambient temperature in CDCl3 with a Bruker Advance 300 instrument (Wissenbourg, France) operating at 300.13 and 75.47 MHz, respectively. EI mass spectra were measured on a Hitachi Perkin-Elmer RMV-6M instrument (Perkin-Elmer, USA). HRMS mass spectra were measured on a Kratos Concept II 2 sector/mass spectrometer (Kratos Analytical, Urmston, Manchester, UK). Rotations were determined on a Polax-2 L instrument (Atago Co., Ltd., Tokyo, Japan). Si gel 60 (0.063–0.200–mm, Merck, Damstadt, Germany) for column chromatography and Si Gel 60 GF254 (Merck, Damstadt, Germany) for analytical and preparative TLC were used.
Plant material
Schisandra verruculosa. Gagnep (Schisandraceae) was collected in Mae Tang, Chiang Mai, northern Thailand, in December 2002. The plant material was identified by Dr. J.F. Maxwell. A voucher specimen (voucher no. 01-103) was deposited at the CMU Herbarium, Faculty of Science, Chiang Mai University, Thailand.
Extraction and isolation
Dried and powdered stem wood of Schisandra verruculosa. (10 kg) was percolated by methanol to exhaustion (3 × 20 l) at room temperature. The methanol solution was evaporated under reduced pressure to give a crude residue (142 g), which was then dissolved in CHCl3(3 × 5 l). The CHCl3 solutions were combined and evaporated at the reduced pressure to give crude chloroform extract (118 g). One part of the crude CHCl3 extract (96 g) was dissolved in warm ethanol (900 ml) to which was added 1 l of H2O containing 34.8 g of lead acetate and 10 ml of glacial acetic acid. The solution was kept in a dark chamber for 48 h and filtered; the filtrate was concentrated at reduced pressure to remove EtOH and extracted with CHCl3 (4 × 250 ml.). The combined CHCl3 extracts were dried (Na2SO4), filtered, and evaporated at reduced pressure to give a syrupy mass (4 g), which was applied to a Si gel column (50 g) and eluted with petrol-CHCl3, CHCl3, CHCl3-Me2CO, 300 ml fractions being collected as follows: Frs. 1–31 (petrol-CHCl3, 4:1), 32–51 (petrol-CHCl3, 3:2), 52–90 (petrol-CHCl3, 3:7), 91–104 (petrol-CHCl3, 1:4), 105–123 (CHCl3), 124–160 (CHCl3-Me2O, 9:1), 161–184 (CHCl3-Me2O, 7:3), 185–197 (CHCl3-Me2O, 1:1). Frs. 57–72 were combined (180 mg) and purified by TLC (Si gel, toluene-EtOAc-CHCl3-HCO2H, 50:35:15:1) to give abscisic acid (3; 20 mg). Frs. 128–146 were combined (330 mg) and purified by TLC (Si gel, CHCl3-Me2O-HCO2H, 85:15:1) to give vanillic acid (8; 12 mg). Frs. 147–162 were combined (330 mg) and purified by TLC (Si gel, CHCl3-Me2O-HCO2H, 85:15:1) to give vanillic acid (8; 8 mg).
Another part of the crude CHCl3 extract (22 g) was applied to a Si gel column (200 g) and eluted with petrol-CHCl3, CHCl3, CHCl3-Me2CO, 500 ml fractions being collected as follows: Frs. 1–5 (petrol-CHCl3, 7:3), 6–75 (petrol-CHCl3, 1:1), 76–130 (petrol-CHCl3, 3:7), 131–150 (petrol-CHCl3, 1:9), 151–165 (CHCl3-Me2O, 9:1)and 166–180 (CHCl3-Me2O, 7:3). Frs. 7–30 (857 mg) were combined and applied to a Si gel column (20 g) and eluted with petrol-CHCl3, 100 ml sfrs. being collected as follows: Sfrs. 1–31 (CHCl3-petrol, 1:1), 32–53 (CHCl3-petrol, 7:3) and 55–66 (CHCl3-petrol, 9:1). Sfrs. 5–8 (23 mg) were combined and purified by TLC (Si gel, CHCl3-petrol-HCO2H, 95:5:1) to give methyl 4-hydroxybenzoate (6; 3.3 mg) and 4-hydroxybenzaldehyde (4; 6.9 mg), respectively. Sfrs. 9–15 (24 mg) were combined and purified by TLC (Si gel, CHCl3-petrol-HCO2H, 95:5:1) to give 4-hydroxybenzaldehyde (4; 8.3 mg). Frs. 36–40 (342 mg) were combined and applied to a Si gel column (15 g) and eluted with petrol-CHCl3, 100 ml sfrs. being collected as follows: Sfrs 1–50 (CHCl3-petrol, 1:1), 51–55 (CHCl3-petrol, 7:3), and 56–65 (CHCl3-petrol, 9:1). Sfrs 5–11 (42 mg) were combined and purified by TLC (Si gel, CHCl3-Me2O-HCO2H, 85:15:1) to give methyl 3,4-dihydroxybenzoate (7; 23 mg) and 2 (9.6 mg). Frs. 61–97 (560 mg) were combined and applied to a Si gel column (15 g) and eluted with petrol-CHCl3, 100 ml sfrs. being collected as follows: Sfrs 1–47 (CHCl3-petrol, 1:1) and 48–73 (CHCl3-petrol, 7:3). Sfrs. 31–60 (69 mg) were combined and purified by TLC (Si gel, CHCl3-Me2O-HCO2H, 85:15:1) to give methyl 3,4-dihydroxybenzoate (7; 11 mg) and 1 (9.5 mg). Frs. 131–160 (545 mg) were combined and applied to a Si gel column (15 g) and eluted with petrol-CHCl3, 200 ml sfrs. being collected as follows: Sfrs. 1–22 (CHCl3-petrol, 7:3), 23–34 (CHCl3-petrol, 9:1), 35–40 (CHCl3-Me2O, 9:1), 41–50 (CHCl3-Me2O, 4:1). Sfrs. 24–35 (74 mg) were combined and purified by TLC (Si gel, CHCl3-MeOH-HCO2H, 96: 4:1) to give 4-hydroxybenzoic acid (5; 15 mg).
1,2-bis-(4-Hydroxy-3-methoxyphenyl)-3-hydroxypropan-1-one. (1): White viscous mass, EI-HRMS: M+ 318.11028, calcd. for C17H18O6, 318.11034. [α]D25 = − 16° (CHCl3, c = 1.2 g/100 ml), 1H NMR (CDCl3, 300 MHz): 7.54 d (J. = 1.9, H-2′), 7.53 dd (J. = 8.8, 1.9, H-6'), 6.86 d (J. = 8.0, H-5″), 6.84 d (J. = 8.8, H-5′), 6.80 dd (J. = 8.0, 1.9, H-6″), 6.71 d (J. = 1.9, H-2″), 4.66 dd (J. = 8.2, 5.0, H-2), 4.22 dd (J. = 11.3, 8.4, H-3), 3.82–3.89 (under the OMe peak, H-3), 3.90 s (OMe-3′), 3.84 s (OMe-4″); 13C NMR (CDCl3, 75.47 MHz): 198.6 (C-1), 150.5 (C-4′), 146.9 (C-3′), 146.5 (C-3′), 145.1 (C-4″), 129.1 (C-1′), 128.4 (C-1″), 124.5 (C-6′), 121.5 (C-6″), 111.0 C-5″), 113.9 (C-5′), 110.5 (C-2′), 110. 1 (C-2″), 65.3 (C-3), 55.9 (OMe), 55.5 (C-2).
1-(4-Hydroxy-3-methoxyphenyl)-3-hydroxypropan-1-one. (2): 1H NMR (CDCl3, 300 MHz): 7.57 d (J. = 7.8, H-6′), 7.54 s (H-2′), 6.96 d (J. = 7.8, H-5′), 6.14 brs (OH), 4.03 t (J. = 5.3, H-3), 3.97 s (OMe), 3.19 t (J. = 5.3, H-2); 13C NMR (CDCl3, 75.47 MHz): 199.1 (C-1), 150.8 (C-4′), 146.7 (C-3′), 129.6 (C-1′), 123.7 (C-6′),113.9 (C-2′), 109.5 (C-5′), 58.3 (C-3), 56.1 (OMe), 39.73 (C-2).
Abscisic acid. (3): Yellow viscous mass. 1H NMR (CDCl3, 300 MHz): 7.38d (J. = 16.0, H-4), 6.16 d (J. = 16.0, H-5), 5.96 brs (H-3′), 5.78 brs (H-2), 2.49 d (J. = 17.0, H-5′), 2.30 d (J. = 17.0, H-5′), 2.40 d (J. = 1.0, Me-3), 1.93d (J. = 1.2, Me-2′), 1.12 s (Me-6′), 1.03 s (Me-6′). 13C NMR (CDCl3, 75.47 MHz): 198.0 (C-4′), 170.3 (C-1), 162.6 (C-2′), 151.6 (C-3), 136.9 (C-5), 128.1 (C-4), 127.1 (C-3′), 117.8 (C-2), 79.8 (C-1′), 49.7 (C-5′), 41.6 (C-6′), 24.3 (Me-6′), 23.8 (Me-6′) 21.4 (Me-3), 19.0 (Me-2′). + FAB HRMS (in NBA): M + H+, 247.13338, calcd. for C15H19O3, 247.13342.
Biological activity assays
Chemicals
Ascorbic acid, cyclosporin A, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), 1,1-diphenyl-2-picrylhydrazyl (DPPH), dimethylsulfoxide (DMSO), doxorubicin hydrochloride, gentamicin, L-glutamine, Histopaque-1077, phytohemagglutinin (PHA), and sulforhodamine B (SRB) were purchased from Sigma Chemical Co. (St. Louis, USA) Ethanol was obtained from Merck Ltd. Fetal bovine serum (FBS) and RPMI-1640 medium were obtained from Gibco BRL (Scotland, UK). National Cancer Institute (NCI; Bethesda, MD, USA) kindly provided the tumor cell lines.
Tumor cell growth assay
Stock solutions of compounds in DMSO were prepared and stored at − 20°C. The frozen samples were diluted with cell cultures medium immediately prior to the assay. Final concentrations of DMSO (≤ 0.25%) did not interfere with tumor cell growth. The effect of compounds on the growth of human tumor cell lines were evaluated according to the procedure adopted by the NCI for in vitro. anticancer drug screening that uses the protein-binding dye SRB to assess cell growth (Skehan et al., Citation1990). Three human cell lines, MCF-7 (breast adenocarcinoma), NCI-H460 (non-small cell lung cancer), and SF-268 (CNS cancer), were used. Cells were routinely maintained as adherent cell cultures in RPMI-1640 medium supplemented with 5% heat-inactivated FBS, 2 mM glutamine, and 50 µg/ml of gentamicin at 37°C in a humidified air incubator containing 5% CO2. Each cell line was plated at a density that ensured exponential growth throughout the experimental period according to their growth profiles (7.5 × 104 cells/ml for NCI-H460 and 1.5 × 105 cells/ml for MCF-7 and SF-268) in 96-well plates and allowed to attach overnight. Cells were then exposed for 48 h to five serial concentrations of compounds. After this incubation period, the adherant cells were fixed in situ., washed, and dyed with SRB. The bound stain was solubilized, and the absorbance was measured at 492 nm in a microplate reader. For each test compound and for each cell line, a dose-response curve was generated and the growth inhibition of 50% (GI50), corresponding with the concentration of compound that inhibits 50% of the net cell growth was determined as described (Monks et al., Citation1991). Doxorubicin was used as positive control.
Human lymphocytes proliferation assay
The effect of compounds on the mitogenic response of human lymphocytes to PHA were evaluated using a modified colorimetric MTT assay (Mosman, 1983), previously described by our group (Gonzalez et al., Citation1999). Human mononuclear cells were isolated from heparinized peripheral blood of healthy volunteers by Histopaque-1077 density centrifugation and were adjusted to 2 to 3 × 106 cells/ml in RPMI-1640 supplemented with 10% FBS, 2 mM glutamine, and 50 µg/ml of gentamicin. Mononuclear cells in 96-well plates were exposed for 4 days to seven serial concentrations of compounds. After this period, MTT solution (1 mg/ml) was added and plates were incubated for 4 h. The water-insoluble formazan dye was solubilized overnight at 37°C. The absorbance of the colored solution was then measured in a microplate reader at 550 nm. The concentration giving 50% inhibition in the test system (IC50) was calculated. Cyclosporin A was used as positive control. Lymphocytotoxicity, determined in terms of the percentage of viable cells, was present when the viability of the exposed cells was less than 70% of the nonexposed control cells.
DPPH free radical scavenging assay
The free radical scavenging activity of the compounds was determined by a modified DPPH assay (Tachibana et al., Citation1990). Briefly, 75 µl of the ethanol solution of sample and 75 µl of 200 µM ethanol solution of DPPH were placed into each well of a 96-well flat-bottom microplate (final concentration 100 µM). The reaction mixtures were allowed to stand for 30 min at room temperature, and the absorbance was measured at 550 nm against a blank (ethanol). The experiments were done in triplicate. The DPPH free radical scavenging activity was calculated according to the following equation:
The IC50 was defined as concentration of compounds that showed 50% DPPH scavenging activity. Ascorbic acid was used as a positive control.
Results and Discussion
Common plant secondary metabolites such as vanillic acid (8), methyl 3,4-dihydroxybenzoate (5), methyl 4-hydroxybenzoate (6), 4-hydroxybezaldehyde (7), 4-hydroxybenzoic acid (5), abscisic acid (3), and two phenylpropanoids, 1,2-bis.-(4-hydroxy-3-methoxy-3-hydroxypropan-1-one (evafolin B, 1) and 1-(4-hydroxy-3-methoxyphenyl)-3-hydroxypropan-1-one (2), were isolated from the stem wood of Schisandra verruculosa. (). Although compounds 1 and 2 were isolated for the first time from the plants of the genus Schisandra., they have been previously reported as constituents of Tetradium glabrifolium. (Champ. Ex Benth) T. Hartley (Rutaceae) (Wu et al., Citation1995) and Bauhenia manca. Standley (leguminosae) (Achenbach et al., Citation1988), respectively.
Compounds 1–8 were evaluate for their capacity to inhibit growth of human tumor cell lines: MCF-7 (breast), NCI-H460 (lung), and SF-268 (CNS), and on the proliferation of human lymphocytes () as well as for their scavenging capacity for DPPH free radicals (). The results showed that only methyl 3,4-dihydroxybenzoate (7) exhibited moderate inhibitory activity on the three tumor cell lines and on human lymphocyte proliferation. Interestingly, compound 7 also showed a strong scavenging activity for DPPH free radical, only slightly lower than ascorbic acid.
Table 1. Effects of compounds 1–8 on the growth of human tumor cell lines and proliferation of human lymphocytes (Gonzalez et al., Citation1999).
Table 2. Effects of compounds 1–8 on DPPH free radical scavenging activity.
Acknowledgments
This investigation was supported by Fundação para a Ciência e Tecnologia of Portugal (Unidade de I&D 226/96), POCTI (QCA III), FEDER, CIIMAR Plurianual. Rujida Wilairat thanks Thailand Research Fund (TRF) for a fellowship under the RGJ-PhD program.
References
- Achenbach H, Stocker M, Constenla MA (1988): Flavonoid and other constituents of Bauhenis manca.. Phytochemistry 27: 1835–1841. [CSA]
- Chen YG, Qin GW, Cao L, Leng Y, Xie YY (2001): Triterpenoid acids from Scisandra propinqua. with cytotoxic effect on rat luteal cells and human decidual cell in vitro.. Fitoterapia 72: 435–437. [INFOTRIEVE], [CSA], [CROSSREF]
- Chen YG, Wu ZC, Gui SH, Lv YP, Liao XR, Halaweish F (2005). Lignans from Schisandra henryi. with DNA cleaving activity and cytotoxic effect on leukaemia and Hela cells in vitro.. Fitoterapia 76: 370–373. [INFOTRIEVE], [CSA], [CROSSREF]
- Fujihashi T, Hara H, Sakata T, Mori K, Higuchi H, Tanaka A, Kaji H, Kaja A (1995): Anti-human immunodeficiency virus (HIV) activities of halogenated gomisin J derivatives, new nonnucleoside inhibitors of HIV type 1 reverse transcriptase. Antimicrob Agents Chemother 39: 2000–2007. [INFOTRIEVE], [CSA]
- Gonzalez MJ, Nascimento MSJ, Cidade HM, Pinto MMM, Kijjoa A, Anantachoke C, Silva AMS, Herz W (1999): Immunomodulatory activity of xanthones from Calophyllum teymannii. var. inophylloide.. Planta Med 65: 368–371. [CSA]
- Hancke JL, Burgos RA, Ahumada F (1999): Schsandra chinensis. (Turcz.) Baill. Fitoterapia 70: 451–471. [CSA], [CROSSREF]
- Jiaxiang N, Fujii K, Sato N, Yuge O (1993): Inhibitory effect of gomisin on reductive metabolism of halothane. Appl Toxicol 13: 385–388. [CSA]
- Kuo YH, Kuo LMY, Chen CF (1997): Four new C19 homolignans, schiarisanrins A, B, D and cytotoxic schiarisanrin C, from Schisandra arisanensis.. J Org Chem 62: 3242–3245. [INFOTRIEVE], [CSA], [CROSSREF]
- Li RT, Li SH, Z QS, Lin ZW, Sun HD, Lu Y, Wang C, Zheng QT (2003): Lancifodilactone A, a novel bisnortriterpenoid from Schisandra lancifolia.. Tetrahedron Lett 44: 3531–3534. [CSA], [CROSSREF]
- Li R, Shen Y, Xiang W, Sun H (2004a): Four novel nortriterpenoids isolated from Schisandra henryi. var. yunnanensis.. Eur J Org Chem 4: 807–811. [CSA], [CROSSREF]
- Li L, Ren HY, Yang XD, Zhao JF, Li GP, Zhang HB (2004b): Rubriflorin A and B, two novel partially saturated dibenzocyclooctene lignans from Schisandra rubriflora.. Hel Chim Acta 87: 2943–2947. [CSA], [CROSSREF]
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, Gray-Goodrich M, Campbell H, Mayo J, Boyd M (1991): Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83: 757–765. [INFOTRIEVE], [CSA]
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63. [INFOTRIEVE], [CSA], [CROSSREF]
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd M (1990): New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82: 1107–1112. [INFOTRIEVE], [CSA]
- Sun HD, Qiu SX, Lin LZ, Wang ZU, Lin ZW, Pengsuparp T, Pezzuto JM, Fong HHS, Cordell GA, Farnsworth NR (1996): Nigranoic acid, a triterpenoid from Schisandra sphaerandra. that inhibits HIV-1 reverse transcriptase. J Nat Prod 59: 525–527. [INFOTRIEVE], [CSA], [CROSSREF]
- Tachibana Y, Kikuzaki H, Lajis NH, Nakatani N (1990): Antioxidative activity of cabazoles from Murraya koenigii. leaves. J Agric Food Chem 48: 5589–5594. [CSA]
- Wu TS, Yeh JH, Wu PL (1995): The heartwood constituents of Tetradium glabrifolium.. Phytochemistry 40: 121–124. [CSA], [CROSSREF]