Abstract
The 80% methanol of the pericarp of the fruits of Adansonia digitata. L. (Bombaceae) was found to contain proanthocyanidins as major compounds, vi.z., (-)-epicatechin, epicatechin-(4 → β.8)-epicatechin B2, epicatechin-(4 → β.6)-epicatechin B5, epicatechin-(2β. → O → 7, 4β. → 8)-epicatechin A2, and epicatechin-(4 → β.8)-epicatechin-(4 → β.8)-epicatechin C1. All the isolated compounds were isolated by open-column liquid chromatography (CC) using Sephadex-LH 20 as stationary phase. Elution of the column was performed with EtOH. The structures of these compounds were elucidated by means of TLC, ESI-MS, and 1D and 2D NMR spectroscopy.
Introduction
The genus Adansonia. (Bombaceae) (bombax or baoboab family) is known by various names such as baobab, dead-rat tree, bottle tree, Monkey-bread tree, and lemonade tree. The genus Adansonia. comprises eight species; one growing in Australia, one in Africa, and the rest are restricted to the island of Madagascar. Adansonia digitata. L. is endemic to Africa. It is one of the largest and reportedly longest living species (6000 years) of the world (Ramadan et al., Citation1994; Shukla et al., Citation2001). Several reports have indicated the use of A. digitata. in folk medicine as antipyretic, febrifuge, astringent in diarrhea and dysentery, also as substitute for cinchora in various systems of medicine (Ramesh et al., Citation1992). The pith of the fruit contains high levels of ascorbic acid (vitamin C), tartaric acid, and citric acid and is used in producing a refreshing drink. This drink is used medicinally to treat fevers, diarrhea, and hemoptysis (spitting of blood from the lungs). Seeds are eaten fresh or dried. They can also be ground into a powder and used as a substitute for coffee. The leaves are said to be rich in vitamin C, sugars, potassium tartrate, and calcium. The leaves are freshly cooked as a vegetable or dried and crushed for later use by local people (van Wyk et al., 2000; Esterhuyse et al., Citation2001).
The leaves are used medicinally against fever by reducing sweating and as an astringent by tightening mucous membranes thus reducing mucous secretions. In West Africa, the leaves (and bark) are used for treating urinary disorders and diarrhea. Young roots are cooked and eaten (Wickens, Citation1982; van Wyk et al., 2000; Esterhuyse et al., Citation2001). Toxicological and pharmacological, anti-inflammatory, analgesic, and antipyretic effects of the fruit pulp of A. digitata. have been studied. Phytochemical screening of the fruit pulp of the plant gave positive reaction for sterols, triterpenes, saponins, tannins, carbohydrates, and glycosides (Ramadan et al., Citation1994).
A variety of chemicals have been isolated and characterized from A. digitata.. They belong to the classes of terpenoids, flavonoids, sterols, vitamins, amino acids, carbohydrates, and lipids (Shukla et al., Citation2001). A new flavanol glycoside isolated from the roots was characterized as 3,7-dihydroxy-flavan-4-one-5-O.-β.-D-galactopyranosyl (1 → 4)-β.-D-glucopyranoside (Chauhan et al., Citation1984). A flavanone glycoside isolated from the roots of A. digitata. was characterized as 3,3′,4′-trihydroxy flavan-4-one-7-O.-α.-L-rhamnopyranoside. Quercetin-7-O.-β.-D-xylopyranoside was also isolated from the roots of this plant (Chauhan et al., Citation1987; Shukla et al., Citation2001). This report concerns the isolation and structural elucidation of proanthocyanidin compounds for the first time from Adansonia digitata..
Procyanidins are a class of proanthocyanidins (condensed tannins) consisting of flavan-3-ol units, epicatechins, and/or catechins. Flavanol units are primarily interlinked through C-4/C-8 linkages, but a C-4/C-6 and a double interflavanoid linkage (C–C and C–O) may also exist. Procyanidins can be categorized as oligomeric procyanidins (OPs) consisting of 2–6 flavanol units and polymeric procyanidins (PPs) consisting of more than six flavanol units (Haslam, Citation1998). In recent years, attention has been paid to polyphenolic procyanidins as a result of their antioxidant, radical scavenging, antiviral, and anti-HIV activities (Shahat et al., Citation1998Citation2002; Saint-Cricq de Gaulejac et al., Citation1999).
Materials and Methods
General
TLC was carried out on precoated silica gel F254 plates (0.2 mm, Merck, Darmstadt, Germany) developed with EtOAc/HOAc/formic acid/water. Spots were detected using vanillin-H2SO4 (vanillin 1% in methanol and 5% H2SO4 in EtOH) followed by heating the plates to 110°C for 15–20 min. Column chromatography (CC) was performed on Sephadex LH-20 (Pharmacia), and silica gel 60 (230–400 mesh ASTM, Merck). TLC: Silica gel 60 F254, layer thickness 0.2 mm (Merck). Solvent system.: EtOAc-HOAc-HCOOH-H2O (30/0.8/1.2/8) v/v. 1H and 13C NMR spectra were recorded in acetone-d.6 on a Bruker DRX-400 spectrometer operating at 400 MHz for 1H and at 100 MHz for 13C. Chemical shifts are presented in ppm downfield of TMS. Electrospray ionization mass spectra (ESI-MS) were obtained on a Bruker Esquire 3000 plus mass spectrometer.
Plant material
Adansonia digitata. was purchased at the Harraz Herbal Drugstore (Cairo, Egypt) in October 2001 and identified by taxonomist Ibrahim El-Garf, Department of Botany, Faculty of Science, Cairo University, Cairo, Egypt.
Extraction and isolation
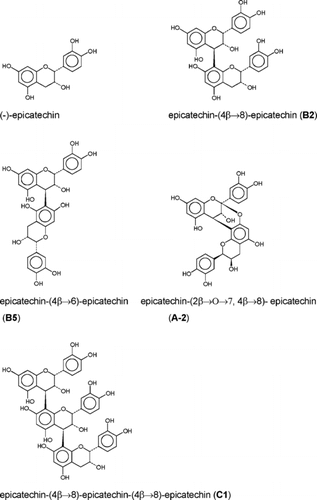
The dried pericarp (200 g) was ground to powder and extracted exhaustively with 80% aqueous MeOH (4 × 500 ml). The combined extract was concentrated on a rotatory evaporator at 35°C under reduced pressure and the residue was diluted with 20% aqueous MeOH and defatted by extracting 2 × with an equal volume of petroleum ether. The aqueous phase was reduced to a smaller volume and extracted 4 × with an equal volume of EtOAc. The EtOAc extract was combined and concentrated to afford 5.2 g of solid. Five g of the solid were dissolved in small amount of methanol and chromatographed over a Sephadex LH-20 column using EtOH as the eluting solvent. Fractions (12 ml) were collected with fraction collector and monitored by TLC. On the basis of the TLC results, fractions were collected to give five subfractions (I–VI). Fractions V and VI will be investigated later. Repeated column chromatography of fractions I–IV over Sephadex LH-20 with methanol or ethanol yielded quercetin-3-O.-β.-D-glucoside (1), epicatechin (2), epicatechin-(4 → β.8)-epicatechin B2 (3), epicatechin-(4 → β.6)-epicatechin B5 (4), epicatechin-(2β. → O → 7, 4β. → 8)-epicatechin A2 (5), and epicatechin-(4 → β.8)-epicatechin-(4 → β.8)-epicatechin C1 (6) ().
Compound 2, (-)-epicatechin
Compound 2 was an off-white amorphous solid, TLC Rf = 0.87 positive ES-MS: m./z. 313 [M + Na]+, 391 [M + H]+, 1H NMR (400 MHz, acetone-d.6) δ. 6.97 (1 H, d, J. = 1.7 Hz, H-2′); 6.78 (1 H, dd J. = 7.5, 1.7 Hz, H-6′); 6.74 (1 H, d, J. = 7.5 Hz, H-5′); 5.94 (1 H, d, J. = 2.2 Hz, H-6) 5.91 (1 H, d, J. = 2.2 Hz, H-8); 4.81 (H-2, s); 4.17 (H-3, broad s); 2.85 (1 H, dd, J. = 4.5, 16.5 Hz, α. H-4); 2.73 (1 H, dd J. = 2.5, 16.7 Hz, β. H-4).
13C NMR (100 MHz, acetone-d.6) δ. 29.22 (C-4); 67.74 (C-3); 79.85 (C-2); 95.92 (C-8); 96.46 (C-6); 100.09 (C-10); 115.34 (C-2′) 115–92 (C-5′); 119.12 (C-6′); 132.30 (C-1′); 145.72 (C-4′); 145.89 (C-3′); 157.34 (C-9); 157.61 (C-7); 157.95 (C-5).
Compound 3 (B2)
Compound 3 was a single spot on TLC (Rf = 0.78) changing to red after spraying and heating (characteristic for procyanidins). ES-MS (positive ion mode) m./z.: 601 ([M + Na]+) and 579 [M + H]+.
1H NMR (400 MHz, acetone-d.6) δ. 7.01–5.06 for the aromatic ring B and A (H-2′, H-6′, H-5′; H-6, H-8), 5.10 (H-2u, br s); 4.96 (H-2t, br s), 4.01 (H-3u, broad s); 4.34 (H-3t, broad s); 4.73 (H-4u, broad s); 2.91 (4t α., dd, 16.7 Hz, 4.5 Hz); 2.74 (4tβ., dd, J. = 16.7 Hz, 4.5 Hz).
Compound 4 (B5)
Compound 4 was a single spot on TLC (0.82) changing to red after spraying and heating (characteristic for procyanidins). ES-MS (positive ion mode) m./z.: 601 [M + Na]+and 579 [M + H]+.
1H NMR (400 MHz, acetone-d.6) δ. 6.90–5.06 for the aromatic ring B and A, H-2′, H-6′, H-5′ H-6, H-8), 4.95 (H-2u, br s); 4.82 (H-2t, br s), 4.06 (H-3u, broad s); 4.15 (H-3t, broad s); 4.64 (H-4u, broad s); 2.78 (4tα., br d); 2.64 (4tβ., br d).
Compound 5 (A2)
Compound 5 was a single spot on TLC changing to red after spraying and heating (characteristic for procyanidins). ES-MS (positive ion mode) m./z.: 599 ([M + Na]+) and 577 [M + H]+.
1H NMR (400 MHz, acetone-d.6) δ. 7.29–6.76 (aromatic ring B, H-2′, H-6′, H-5′), 6.12–5.95 (aromatic A, H-6, H-8), 4.95 (H-2t, br s); 4.14 (H-3u, d, J. = 3.2); 4.32 (H-3t, d, J. = 3.2); 4.33 (H-4u, d, J. = 3.2); 2.95 (4tα., dd, J. = 17.2, 4.8); 2.80 (H-4tβ., br d, 17.2).
Compound 6 (C1)
Compound 6 was a pale brown amorphous powder showing single spot on TLC (Rf = 0.65) changing to red after spraying and heating. ES-MS (positive ion mode) m./z.: 889 ([M + Na]+) and 867 [M + H]+.
13C NMR data of compound 5 (100 MHz, acetone-d.6): 158.28–153.90 (C-5; C-7; C-9); 145.23–144.89 (C-3′, C-4′); 132.20–131.76 (C1′); 118.78 (C-6′); 115.44 (C-5′); 114.80 (C-2′); 107.13 (C-8t); 106.84 (C-8m); 102.10 (C-10 u, C-10 m); 100.45 (C-10t); 97.13 (C-6m, C-6t); 96.41 (C-6u); 95.85 (C-8u); 79.00 (C-2t); 76.78 (C-2u): 76.63 C-2m); 72.93 (C-3u); 71.86 (C-3m); 66.30 (C-3t); 36.81 (C-4m, C-4u); 28.88 (C-4t).
Results and Discussion
Confirmation of the proanthocyanidin constitution of the ethyl acetae fraction was made by TLC and heating after spraying with vanillin/sulfuric acid, which yielded cyanidin as the principle pigment.
Compound 1 was identified as quercetin-3-O.-β.-D-glucoside by comparison of its physical and spectral data and co-chromatography with those of an authentic sample (Markham, Citation1989).
Compound 2 was isolated as an off-white amorphous powder, positive to vanillin/sulfuric acid. Positive ESI-MS exhibited a sodiated molecular ion peak [M + H]+ at m./z. 313 suggesting a monomer (catechin or epicatechin). The 1H NMR spectrum of 2 showed two doublets of doublets at δ. 2.85 and 2.73 assigned to the H-4 protons (coupled to each other with J. = 16.7 Hz, and to H-3 with J. = 4.5 and 2.5 Hz) and a singlet at δ. 4.81 (H-2, s) and δ. 4.17 (H-3, broad s). Aromatic signals appeared at δ. 6.97 (1 H, d, J. = 1.7 Hz, H-2′), 6.78 (1H, dd, J. = 7.5, 1.7 Hz, H-6′), and 6.74 (1H, d, J. = 7.5 Hz,H-5′) and as a pair of meta-coupled doublets (J. = 2.2 Hz) at δ. 5.94 (H-6) and δ. 5.91 (H-8). 1H NMR suggested an epicatechin structure. This was confirmed by the presence of two signal peaks in 13C NMR at δ. 79.85 and 67.74 for C-2 and C-3, respectively (Porter et al., Citation1982).
Compound 3 showed [M + Na]+ and [M + H]+ peaks at m./z. 601 and m./z. 579 in positive ESI-MS, indicating a molecular weight of 578, which is in agreement with a B-type procyanidin dimer. According to a systematic overview of the 1H and 13C NMR assignments of the B-type procyanidins by De Bruyne et al. (Citation1999), the chemical shifts of the heterocyclic protons and carbons indicated an epicatechin-epicatechin dimer with a 4β.–8 linkage. The heterocyclic ring carbon shifts at δ. 79.17 and 76.86 of C-2 (t) and C-2 (u), respectively, point to two epicatechin units. The epicatechin C-2 carbon signal at δ. 76.86 is consistent with a 2,3-cis.-3,4-trans. stereochemistry (Foo & Karchesy, Citation1989). The signals at δ. 145.10–145.38 and at δ. 155.73–158.19 arise from the deshielded oxygen bearing carbons 3′, 4′, and 5, 7, and 9, respectively. The remaining quaternary signals at δ. 100.54–107.01 and 131.96–132.70 are due to C-10, C-8t, and C-1′. The aromatic CH resonances at δ. 95.90–97.01, 115.17–115.45, and 119.07–119.26 are assigned to C-6 and C-8u, C-2′, C-5′, and C-6′. Hence, the compound could be identified as procyanidin B2 or epicatechin-(4β.-8)-epicatechin.
The positive ESI-MS of compound 4 showed [M + Na]+ and [M + H]+ peaks at m./z. 601 and m./z. 579, respectively, similar to compound 3, indicating a molecular weight of 578. The different Rf values on silica gel plates presented by compound 3 (procyanidin B2) (Rf = 0.78) and compound 4 (procyanidin B5) (Rf = 0.82) suggested a different mode of linkage. The 13C NMR spectral data of compound 4 () were in good agreement with those of a procyanidin consisting of two epicatechin units (C-2 and C-3 chemical shifts at δ. 76.96 and 72.04 for the upper unit and δ. 79.17 and 66.79 for the lower unit, respectively) (Fletcher et al., Citation1977). The downfield shift of C-6 (δ. 107.95) of compound 4 compared to procyanidin B2 indicated a (4 → 6) linkage. Similarly, compound 4 could be identified as procyanidin B5 or epicatechin-(4β.-6)-epicatechin.
Table 1. 13C NMR data of compound 3 (100 MHz, acetone-d.6)
Table 2. 13C NMR data of compound 4 (100 MHz, acetone-d.6)
Compound 5 was isolated as a pale brown amorphous powder and responded positively to the vanillin-sulfuric acid reagent. ESI-MS (positive ion mode) of compound 5 produced a [M + Na] peak at m./z. 599, corresponding to a biflavonoid structure and consistent with a proanthocyanidin A-type with a molecular weight of 576. The presence of the isolated AB coupling system at 4.14–4.34 with J.3,4 = 3.2 Hz was described as a diagnostic feature of the C-ring protons of A-type proanthocyanidins (Lou et al., Citation1999). This was confirmed by the presence of 1 methylene, 13 methines, and 16 quaternary carbons in the 13C NMR spectrum and the molecular weight of 576 as established by ES-MS. The chemical shift of the ketal carbon (C-2) formed as a result of additional bond observed at 99.80 ppm provided further support for A-type linkage. Finally, the 1H and 13C NMR signals were in agreement with the data published by De Bruyne for procyanidin A2, or epicatechin-(4β.-8, 2β.-O.-7)-epicatechin (De Bruyne et al., Citation1999).
Compound 6 gave a red color on TLC with vanillin-sulfuric acid characteristic for procyanidins. The ESI-mass spectrum (positive ion mode) showed a [M + Na]+peak at m./z. 889 indicating a trimeric procyanidin (M = 866), with single linkages. The triflavonoid constitution of compound 6 was easily deduced from the 13C NMR spectrum (). The upfield signals due to the flavan C-2 (77.78 ppm, 77.64 ppm, and 79.00 ppm) were observed, and the chemical shifts of these signals implied the presence of three epicatechin units both with 2,3 cis. configuration, linked through a C-4, C-8 bond (Foo & Karchesy, Citation1989).
Table 3. 13C NMR data of compound 5 (100 MHz, acetone-d.6)
Acknowledgments
The author is grateful to Prof. Dr. A.J. Vlietinck and Prof Dr. L. Pieters (Department of Pharmaceutical Sciences, University of Antwerp, Belgium) for spectral analysis.
References
- Chauhan JS, Cahturvedi R, Kumar S (1984): A new flavanol glycosoide from the roots of A. digitata.. Planta Med 50: 113. [CSA]
- Chauhan JS, Kumar S, Cahturvedi R (1987): A new flavanone glycoside from th roots of A. digitata.. Nat Acad Sci Lett 10: 77–179. [CSA]
- De Bruyne T, Pieters L, Dommisse R, Kolodziej H, Wary V, Van Den Berghe D, Vlietinck A (1999): NMR characterization and biological evaluation of proanthocyanidins: A systematic approach. In: Gross GG, Hemingway RW, Yoshida T, Branham SJ, eds., Chemistry, Biology, Pharmacology, Ecology. New York, London, Moscow, Kluwer Academic/Plenum Publishers, pp. 193–209.
- Esterhuyse N, von Breitenbach J, Söhnge H (2001): Remarkable Trees of South Africa. Pretoria, Briza Publications.
- Fletcher AC, Porter LJ, Haslam E, Gupta RK (1977): Plant proanthocyanidins. Part 3. Conformational and configurational studies of natural procyanidins. J Chem Soc Perkin Trans I 2: 1628–1637. [CSA], [CROSSREF]
- Foo LY, Karchesy JJ (1989): Procyanidin dimers and trimers from Douglas fir inner bark. Phytochemistry 28: 1743–1747. [CSA], [CROSSREF]
- Haslam E (1998): Practical Polyphenolics from Structure to Molecular Recognition and Physiological Action. New York, Cambridge University Press.
- Karchesy JJ, Hemingway RW, Foo LY, Barofsky L, Barofsky DF (1986): Sequencing procyanidin oligomers by fast atom bombardment mass spectrometry. Anal Chem 58: 2563–2567. [CSA], [CROSSREF]
- Lou H, Yamazaki Y, Sasaki T, Uchida M, Tanaka H, Oka S, (1999): A-type proanthocyanidins from peanut skins. Phytochemistry 51: 297–308. [CSA], [CROSSREF]
- Markham KR (1989): Flavones, flavonols and their glycosides. In: Harborne JB, ed., Methods in Plant Biochemistry, Vol. 1. London, Plant Phenolics. Academic Press, pp. 197–235.
- Porter LJ, Newman RH, Foo LY, Wong H (1982): Polymeric proanthocyanidins, 13C N.M.R. studies of procyanidins. J Chem Soc Perkin Trans 1: 1217–1221. [CSA], [CROSSREF]
- Ramadan A, Harraz FM, El-Mougy SA (1994): Anti-inflammmatory, analgesic and antipyretic effects of the fruit pulp of Adansonia digitata.. Fitoterapia 65: 418–422. [CSA]
- Ramesh D, Dennis TJ, Shingare MS (1992): Constituents of Adansonia digitata. root balk. Fitoterapia 63(3): 278–279. [CSA]
- Saint-Cricq de Gaulejac N, Provost C, Vivas N (1999): Comparative study of polyphenol scavenging activities assessed by different methods. J Agric Food Chem 47: 425–431. [INFOTRIEVE], [CSA], [CROSSREF]
- Shahat AA, Cos P, De Bruyne T, Apers A, Hammouda FM, Ismail S, Azzam S, Cleays M, Goovaerts E, Pieters L, Vanden Berghe D, Vlietinck AJ (2002): Antiviral and antioxidant activity of flavonoids and proanthocyanidins from Crateagus sinaica. Boiss. Planta Med 68: 539–541. [INFOTRIEVE], [CSA], [CROSSREF]
- Shahat AA, Ismail SI, Hammouda FM, Azzam S, Lemiere G, De Swaef S, De Bruyne T, Pieters L, Vlietinck AJ (1998): Anti-HIV activity of flavonoids and proanthocyanidins from Crataegus sinaica.. Phytomedicine 5(2): 133–136. [CSA]
- Shukla YN, Dubey S, Jain SP, Kumar S (2001): Chemistry, biology and uses of Adansonia digitata.-A review. J Med Aromatic Sci 23: 429–434. [CSA]
- van Wyk B, van Oudtshoorn B, Gericke N (2005): Medicinal Plants of South Africa. Pretoria, Briza Publication.
- Wickens GE (1982): The baobab—Africa's upside-down tree. Kew Bulletin 37: 173–209. [CSA]