Abstract
Toxic effects of the ethyl acetate root extract of Dennettia tripetala. G. Baker (Annonaceae) were studied using in vivo. and in vitro. models. The LD50 of the extract was 1120 mg/kg, i.p. No remarkable change was observed in the major organs on postmortem examination, except for the hyperemia and petechiation of the mucous and serous membranes of gastrointestinal and respiratory tracts. The LC50 of 83.96 ppm and estimated ED50 of 8.4 ppm (95% confidence interval) were obtained following a brine shrimp lethality test. The chronic exposure of mice to the extract showed a significant (p < 0.05) increase in packed cell volume (PCV) and red blood cell (RBC) count. The plant extract (0.5–2.0 g extract/kg feed) also produced a dose- and time-related significant (p < 0.05) reduction in the total white blood cell (WBC) count. The total number of lymphocytes and neutrophils were reduced. There was significant (p < 0.05) decrease in the relative organ weight (ROW) of liver on day 63 for all the treatment groups and spleen for the mice that received 2.0 g extract/kg feed. The extract produced a significant (p < 0.05) increase in ROW of the heart on day 63 in mice fed 2.0 g extract/kg diet. The vascular effects of the extract include degenerative, necrotic, and regenerative changes with cellular (mainly mononuclear leukocytes) infiltration in the portal areas of the liver, which was both dose- and time-dependent. The kidneys showed areas of hyperemia, hemorrhages, tubular epithelial degeneration, and necrosis with mononuclear leukocytes infiltrating the perivascular spaces and interstices.
Introduction
The toxicity of some antidiabetic medicinal plants has been reported. Species known to contain toxic constituents such as pyrrolizidine alkaloids are considered toxic. Many plants used to treat diabetes or shown experimentally to be hypoglycemic have toxic effects unrelated to their desired effect (Marles & Farnsworth, Citation1994). Generally, little is known about chronic toxicity of medicinal plants. Because diabetes mellitus is a chronic and insidious condition with no known cure, antidiabetic drugs/plant extracts must be taken for the lifetime of the patient. It is therefore strongly recommended that chronic toxicity studies be performed before recommending a plant-derived drug for antidiabetic therapy (Marles & Farnsworth, Citation1994).
Dennettia tripetala. G. Baker (Annonaceae), the only species in the genus Dennettia., is commonly known as pepper fruit in Nigeria (Egwunyenga et al., Citation1998). It is eaten raw by the people of eastern Nigeria for its pungency, while others use it as a food condiment and for medicinal purposes (Okonkwo & Okoye, Citation1996). Much is not known about its folkloric uses, but the root decoction of the plant is used in the management of clinical signs of diabetes mellitus. Anaga et al. (Citation2006) reported that the ethyl acetate fraction of D. tripetala. caused a concentration-dependent increase of glucose uptake in 3T3-L1 adipocytes with maximal effect at 5.0 µg/ml. Also, the antidiabetic properties of the ethyl acetate root extract of D. tripetala. have been reported in normoglycemic and hyperglycemic rat models (Anaga & Asuzu, Citation2006), confirming the rationale for the folkloric use.
The current study was designed to investigate the possible toxic effects of the ethyl acetate root extract of D. tripetala. using acute and chronic toxicity models in mice, as the decoction of the plant is taken by the diabetic patients throughout their lifetime.
Materials and Methods
The experimental protocol used in this study was approved by the Ethics Committee of the University of Nigeria, Nsukka, and conforms with the guide to the care and use of animals in research and teaching of University of Nigeria (Nsukka, Enugu State, Nigeria).
Plant collection and extraction
D. tripetala. root was collected in April 1998 in Ovoko in Nsukka local government area and identified by Mr. A.O. Ozioko of the Bioresources and Diversity Conservation Program (BDCP); Enugu State, Nigeria). A voucher sample (DBH340T) was kept in the herbarium of the Department of Botany, University of Nigeria, Nsukka.
The plant sample was cut into small bits and dried at room temperature on the laboratory bench. The dried sample was pulverized using a hammer mill. About 500 g of the powdered sample was extracted by cold percolation over 24 h at room temperature using ethyl acetate. The extract was concentrated in vacuo. and the yield was determined. The extract was stored at 4°C until use.
Acute toxicity test
Thirty albino Wister mice (17–38 g) of both sexes obtained from the Laboratory Animal Unit of the Faculty of Veterinary Medicine Farm, University of Nigeria, Nsukka, was used for the study. They were randomly divided into five groups of six mice each. Each group was kept in a clean stainless steel cage and was provided with water and feed ad libitum.. The mice in the various groups were injected intraperitoneally with increasing doses (100, 200, 400, 800, and 1600 mg/kg) of the ethyl acetate extract of D. tripetala., while the control group received an equal volume of distilled water. The mice were allowed access to feed and water ad libitum. and were observed for mortality and toxic signs for 48 h. Lethal dose (LD50) of the extract was determined by Probit transformation (Miller & Tainter, 1957).
Brine shrimp lethality test
The method of Meyer et al. (Citation1982), modified by McLaughlin et al. (Citation1991), was used to study the cytotoxicity of D. tripetala. extract. Briefly, Artemia salina. eggs obtained from a pet shop in Davis, California, were incubated in natural seawater (from Bar Beach, Lagos, Nigeria) in a dam-well under room condition. Forty-eight–hour nauplii (10 in each well) were incubated in known concentrations (75, 125, 250, 500, and 1000 ppm) of the extract for 24 h. All tests were conducted in triplicate. The dead nauplii were subtracted from 10 and the data were analyzed using Probit analysis (Finney Program, DOS) to determine the LC50 at 95% confidence interval. Weak nauplii were noted as an indication of central nervous system depression. The control group was incubated in 0.5% DMSO, the solubilizing agent.
Chronic toxicity of the ethyl acetate extract of D. tripetala. in mice
Sixty-four albino Wistar mice (20–36 g) of both sexes were used for the study. The mice were obtained from the Laboratory Animal Unit of the Faculty of Veterinary Medicine Farm, University of Nigeria, Nsukka. They were randomly placed into 4 groups of 16 mice each.
The extract was incorporated in the feed at 0.5, 1.0, 2.0 g/kg feed, using 10% corn starch as a binder to form the test diet. The feed was pelleted using pelleting equipment after it was thoroughly mixed with water. The pellets were dried at 40°C for 3 days to prevent moldiness.
After 1 week of stabilization, the mice were fed the test diet for 13 weeks. Mice in group 1 continued on the control diet (without the extract), while mice in groups 2, 3, and 4 received 0.5, 1.0, and 2.0 g/kg feed, respectively. Water and feed (test and control) diet were given to the mice ad libitum. throughout the 13 weeks of experimental study. At the end of the 3rd, 6th, 9th, and 13th weeks of the diet treatment, blood samples were collected from the medial cantus of the eye of each mouse in all the groups and into bottles containing anticoagulants for hematology. Three mice were sacrificed from each group for pathologic study. Organs (heart, kidney, spleen, lung, liver, and brain) collected from each sacrificed mouse were blotted dry and weighed. The relative organ weight, ROW (weight of organ as a proportion of the total body weight of each mouse), was calculated and compared with the value of the control. The result obtained was subjected to two-way analysis of variance (ANOVA). Differences between the means were tested at 95% confidence level using Student's t.-test. The following examinations were conducted.
Clinical observation
The mice were observed daily for clinical manifestations of toxicity throughout the period of the experiment.
Hematological observation
Blood samples collected in Eppendorf vials containing Na-EDTA as an anticoagulant were kept in the refrigerator at 4°C until analysis.
Heparinized hematocrit tubes were filled with blood and spun in a microhematocrit centrifuge at 15,000 rpm for 5 min to determine the packed cell volume (PCV). Hemoglobin (Hb) was determined using the cyanomethemoglobin method (Schalm et al., Citation1975). The total and differential white cell (WBC) counts were determined by standard techniques (Schalm et al., Citation1975). The result was subjected to two-way ANOVA, while differences between the means were compared using Student's t.-test at 95% confidence interval.
Histopathological examination
The organs were fixed in 10% formol saline and sectioned (5 µm thick) with a microtome after dehydration and embedded in paraffin wax. They were stained with hematoxylin and eosin for examination and interpretation of histopathological changes.
Results
The yield of the ethyl acetate extract of D. tripetala. was 4.4% w/w dry matter. The extract was dirty brown in color with a pasty consistency.
Acute toxicity testing showed that the LD50 of the ethyl acetate extract of D. tripetala. was 1120 mg/kg, i.p. Most treated mice showed nervous signs of depression, clumping together, writhing, and abnormal mobility of the hind legs with arched back. The first death was recorded 4.5 h after intraperitoneal administration of the highest dose (1600 mg/kg), which caused 100% mortality.
The postmortem examination of the dead mice did not reveal any remarkable morphologic changes in the major organs, except for the hyperemia/petechiation of mucous and serous membranes, especially of the gastrointestinal and respiratory tracts. The effect of the ethyl acetate root extract of D. tripetala. on brine shrimp nauplii showed that the LC50 was 83.96 (30.34–119.38) ppm.
Chronic toxicity of the ethyl acetate extract of D. tripetala. in mice showed the following clinical observations: the mice treated with all the doses (0.5, 1.0, 2.0 g/kg) of the extract showed no overt clinical sign throughout the period of the study. They were apparently healthy, clean, alert, and gained body weight (data not shown).
The effects of feeding the crude extract for 13 weeks on the blood parameters (hematology) of mice are presented as follows:
Packed cell volume. (PCV.): The effect of the extract on the PCV of mice is presented in . There was a significant (p < 0.05) increase in the PCV of the mice in groups 2 and 4 (0.5 and 2.0 g extract/kg feed) on day 21 post-treatment compared with the control group.
Table 1. The effects of the ethyl acetate root extract D. tripetala. on packed cell volume (PCV %) of mice.
Red blood cells.: The effect of the ethyl acetate extract of D. tripetala. on the red blood cells of mice showed that there was a significant (p < 0.05) increase in the number of red blood cells from day 21 throughout the period of the study ().
Table 2. The effects of ethyl acetate root extract of D. tripetala. on red blood cell count in mice (no./µl × 106).
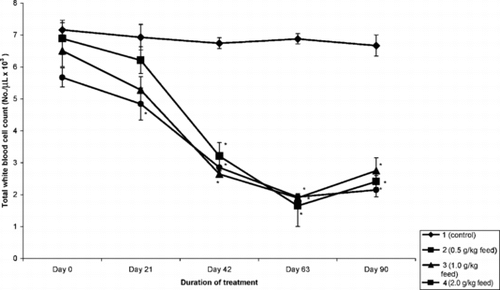
Total and differential white blood cell count. (TWBC.): The effect of the extract on total white blood cell counts is presented in . Two-way ANOVA comparing the means between and within groups showed that there was a significant (p < 0.05) decrease in the number of the total white blood cell (WBC) count beginning from day 21 and lasting until the end of the study.
Figure 1 The effect of the ethyl acetate root extract of D. tripetala. on total white blood cell count in mice.
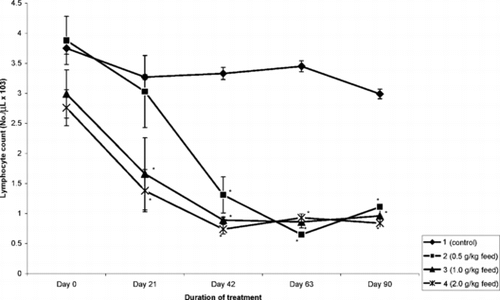
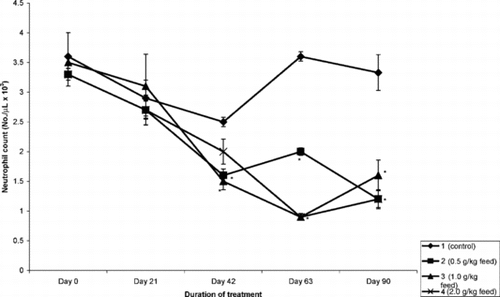
The mean absolute lymphocyte count is presented in . The extract significantly (p < 0.5) reduced the number of lymphocytes in a time- and concentration-dependent manner. The most prominent effect was observed on day 63 of treatment with almost a 60% decease in absolute lymphocyte count. This trend was also observed in the number of monocytes and neutrophils (). However, the effect of the extract on the eosinophils and basophils was inconsistent.
Relative organ weight.: The effects of the ethyl acetate extract of D. tripetala. on relative organ weights of mice are presented in the , a–e.
Table 3. The relative organ weights (mean ± SEM) of mice treated with ethyl acetate extract of D. tripetala. in mice.
There was a significant (p < 0.05) decrease in the relative weight of the liver on day 63 for the mice that received 0.5 and 1.0 g extract/kg feed, and on days 42 and 90 for mice that received the highest dose (2.0 g/kg) of the extract. There was no significant (p > 0.05) decrease in the relative organ weight of the spleen, except for the mice that received 2.0 g of extract/kg feed (, b). The extract significantly (p < 0.05) increased the relative weight of the heart in group 4 mice that received 2.0 g extract/kg feed. Apart from the aberrant results in day 63 of all the treated groups, the extract had no effect on the relative weight of the kidney. However, on day 42 in groups 2 and 4 mice, a significant increase in the weight of the kidney was observed (, d). There was no effect on the relative organ weight of the lungs (data not shown). It also showed no significant effect on the relative organ weight of the brain in all the groups except on day 63.
Histopathologic changes
Liver.: Liver sections collected from the untreated mice (group 1) showed histologic features characteristic of the liver of normal mice. The interlobular connective tissue was scanty, and cords of hepatocytes extend from the central vein to the portal areas. The hepatocytes had centrally vesicular nuclei, with prominent nucleoli (). Sections collected from treated mice showed vascular, degenerative/necrotic, and inflammatory changes at different stages of the treatment period. Sections collected from groups 2 (0.5 g/kg feed) and 3 (1.0 g/kgfeed) mice on day 21 of the treatment showed mild to moderate hyperemia of the hepatic lobule, with moderate degeneration of the hepatocytes and necrosis of a few hepatocytes in the periportal areas (). Sections collected from the two groups by day 63 had mild to moderate vacuolation of hepatocytes, many of which were undergoing necrosis (). Increasing the concentration of the extract given to the mice over a long period of time produced an increase in the severity of the lesions, such that liver sections collected from mice in group 3 (2.0 g/kg feed) on day 42 of treatment showed widespread necrosis of hepatocytes with moderate to severe mononuclear leucocytes infiltration of the portal areas ().
Figure 4 Liver section of untreated (group 1) mouse collected on day 42 of study showing hepatic artery (R) and hepatic portal vein (V) in the portal area. Hepatocytes with vesicular nuclei in cords (arrowheads). H&E stain, ×400.
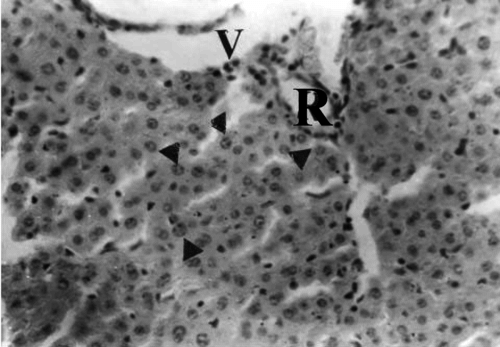
Figure 5 Liver section of mouse in group 3 (1.0 g/kg feed) collected on day 21 of treatment showing hepatocytes undergoing necrosis with pyknotic nuclei (arrowheads) in the portal area with hepatic artery (R). H&E stain, ×400.
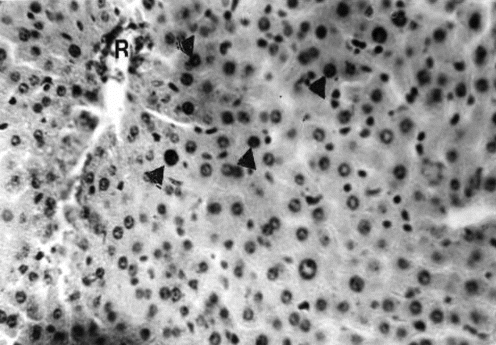
Figure 6 Liver section of mouse in group 3 (1.0 g/kg feed) collected after 63 days of treatment showing widespread vacuolation of hepatocytes and pyknosis of nuclei (arrowhead). H&E stain, ×400.
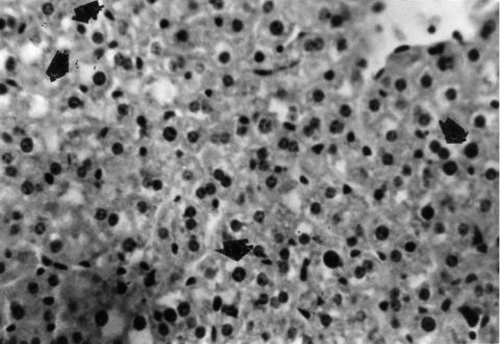
Figure 7 Liver section of mouse in group 4 (2.0 g/kg feed) collected after 42 days of treatment showing widespread necrosis of hepatocytes and many mononuclear leukocytes (arrowheads) around the biliary ductules (B) and the entire portal area. H&E stain, ×400.
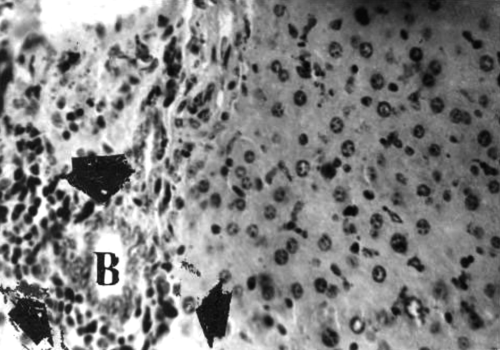
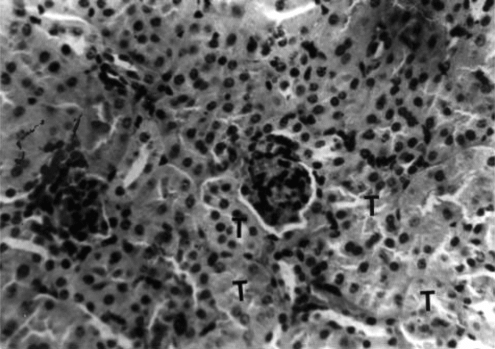
Kidney.: Sections collected from the untreated (group 1) mice throughout the period of the study showed histologic features characteristic of a normal mouse. There were renal corpuscles located exclusively in the cortex, and tubules, located in both the cortex and the medulla (). Kidney sections collected from mice in groups 2 and 3 on days 21 and 42 of treatment showed mild to moderate hyperemia of the cortex and medulla and foci of hemorrhages in the kidney cortex. There was also mild to moderate degeneration of the renal tubules (). Sections collected from mice in group 4 on days 21 and 42 of the treatment showed moderate to severe degeneration and variable necrosis of renal tubules in both cortex and medulla. There was mild to moderate infiltration of the mononuclear leukocytes into the perivascular spaces and interstices ().
Figure 8 Kidney section of untreated (group 1) mouse collected on day 42 of study showing renal corpuscles (arrowheads) and normal epithelial cells lining the tubules (T). H&E stain, ×400.
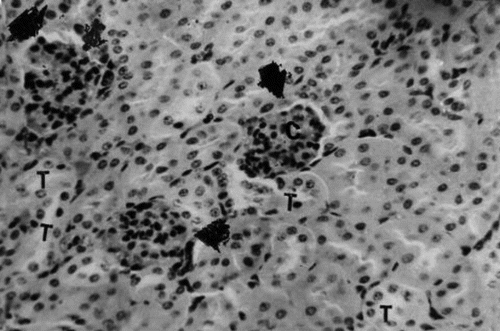
Figure 9 Kidney section of group 3 (1.0 g/kg feed) mouse collected on day 21 of treatment showing degeneration and necrosis of epithelial cells lining the tubules (T). H&E stain, ×400.
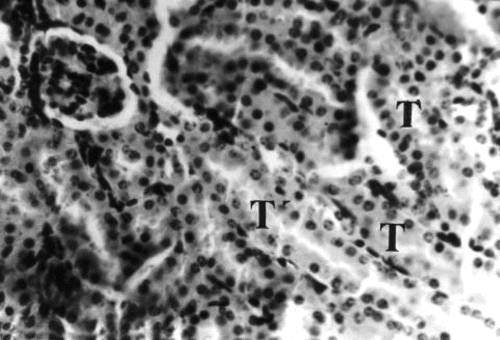
Figure 10 Kidney section of group 4 (2.0 g/kg) mouse collected on day 21 of treatment showing widespread pyknosis of tubular (T) epithelial cells and mononuclear leukocytes infiltration of interstices and perivascular spaces, H&E stain, ×400.
Spleen.: Sections collected from untreated (group 1) mice showed sections with the histomorphology that is characteristic of normal mouse (). Sections collected from group 2 mice at the end of 3 weeks of treatment showed mild congestion of the red pulp and moderately hyperplastic follicles. The red pulp was less congested and follicle was nonreactive by treatment day 63 and appeared depopulated by day 90. Increasing the dose to 1.0 and 2.0 g/kg feed did not appear to have altered the histomorphologic changes from what was observed in group 2 (0.5 g/kg feed) mice throughout the period of observation.
Lungs.: Sections collected from untreated group 1 mice showed normal histologic features of mouse lung, except for evidence of mild hyperemia (). Sections collected from mice fed 0.5 g extract/kg feed showed varying degrees of congestion, characterized primarily by distention of the interalveolar spaces and aggregation of polymorphonuclear (PMN) and mononuclear leukocytes in the peribronchial and peribronchiolar spaces throughout the period of observation (21st to 90th days). Sections from mice fed 1.0 and 2.0 extract/kg feed (groups 3 and 4) showed lesions of advanced interstitial pneumonia between days 63 and 90 (photomicrograph not shown).
Brain.: Sections collected from untreated mice (group 1) showed histologically normal sections. Sections collected from all the treated groups (2, 3, and 4) showed histologic features similar to what was observed in the untreated (group 1) mice, in which the normal histologic features of the different sections of the brain observed from day 21 to day 90 (photomicrograph not shown).
Heart.: Sections from untreated (group 1) mice had normal histology. Mice fed 0.5 g extract/kg feed (group 2) had heart sections with mild myocardial hemorrhages and hyperemic vessels (photomicrograph not shown). These vascular changes increased from day 21 to day 90. Increasing the concentration of the extract in the feed (groups 3 and 4) caused an increase in the severity of the vascular lesions, and in addition, there was mild to moderate myocardial degeneration and necrosis.
Discussion
Little is known about the toxic effect of the ethyl acetate root extract of D. tripetala. in man and animal, except the insecticidal effect reported by Ewete and co-workers (Ewete et al., Citation1996). However, Iwuala et al. (Citation1981) noted that the oil, seeds, and fruits of D. tripetala. appeared to be nontoxic to man and animals. The consumption of the seed oil may be a source of good quality dietary oil for the local people (Breo, Citation1990). Because the root extract of D. tripetala. is effectively used to control diabetes by the natives, it becomes imperative to investigate the extract's short-and long-term toxic effects. The acute toxicity study showed that the crude extract gave an LD50 of 1120 mg/kg, i.p., at 24 h. No gross pathologic change was observed, except catarrhal enteritis. Clinical signs observed in the mice prior to death include depression, abdominal stretching, abnormal gait, and hunched back. Brine shrimp lethality tests gave an LC50 of 83.96 ppm, an indication that the extract contains very potent bioactive compound(s). No death was recorded in the mice treated with various inclusion levels (0.5, 1.0, 2.0 g/kg feed) of the extract throughout the 90-day study period. The treated mice rather showed physical signs of well-being.
However, the blood parameters of the treated mice showed a significant (p < 0.05) increase in the packed cell volume (PCV) and red blood cell (RBC) counts. The increase in RBC count was consistent throughout the period of study, which is probably an indication that the extract increased the rate of erythropoiesis or encouraged hemoconcentration. There may be a correlation between the high content of iron (Anaga et al., 2006) of the plant and the increase in RBC count. Reticulocyte count was, however, not carried out as part of the hematology. Conversely, the crude extract of D. tripetala. significantly (p < 0.05) decreased the total white blood cell count (TWBC) from day 21 to the end of the study. This decrease was reflected on the different white blood cells. The extract significantly (p < 0.05) decreased the number of lymphocytes in a time- and dose-dependent manner, indicating immunosupression. Also, this trend was maintained for monocytes and neutrophils, but the effect on the eosinophils and basophils were inconsistent. These results suggest that the extract has anti-inflammatory properties. The implication of these observations for diabetic patients managed exclusively on this plant extract is the risk of decreased immunity and flare-up of hidden infection or secondary complications. However, there was no secondary infection observed throughout the period of the study in the treated mice.
The effect of the crude extract of D. tripetala. on the relative organ weight showed varied effect from one organ to another. The significant (p < 0.05) reduction in the circulating lymphocytes resulting from treatment correlates very well with the histopathological observation of moderate to severe splenic follicle depopulation, thus explaining the significant (p < 0.05) decrease in the ROW of the spleen.
The histopathologic changes observed in different organs examined were characteristic of circulatory disturbances, degenerative and necrotic changes, and inflammatory reactions. These changes were very prominent in vital organs like liver and kidneys as shown in –10. Because these changes were not observed in the untreated mice (group A), the lesions are attributed to the extract. The severity of the lesions increased as the dose of the extract and duration of treatment were increased, suggesting a cumulative effect of the extract in the tissues. For example, the liver of the extract-treated mice showed mild to moderate congestion, vacuolation, and necrosis of the hepatocytes in the periportal areas. Increasing the dose and time of exposure to the extract led to cellular infiltration (especially mononuclear leucocytes, macrophages, and plasma cells). Generally, the extract must have compromised the integrity of the blood vessels, thereby encouraging extravasations and tissue injury, which in severe cases resulted in necrosis of the cells and the attendant inflammatory reaction.
In conclusion, the extract has a toxic effect, particularly if taken on long time bases. The toxic effect may or may not be ascribed to the active principle of the extract. Therefore, there is need to fractionate the extract in order to isolate the active principle.
References
- Anaga AO, Asuzu IU (2006): Antihyperglycaemic effect of the ethyl acetate fraction of D. tripetala. in vivo.. Fitoterapia (in press). [CSA]
- Anaga AO, Clegg MS, Keen CL, Waterhouse AL, Asuzu IU (2006): Glucose uptake properties of the ethyl acetate fraction of D. tripetala. in 3T3-L1 adipocytes. Phytother Res (in press). [CSA]
- Breo DL (1990): “Phil Sokolof” fights the “fatting” of America. J Am Med Assoc 264: 3071–3073. [CSA], [CROSSREF]
- Egwunyenga OA, Alo EB, Nmorsi OPG (1998): Laboratory evaluation of the repellency of Dennettia tripetala. Baker (Annonaceae) to Dermestes maculates. (F.) (Coleoptera: Dermestidae.). J Stored Prod Res 34(2/3): 195–199. [CSA], [CROSSREF]
- Ewete FK, Arnason JT, Larson J, Philogéne BJR (1996): Biological activities of extracts from traditionally used plants against the European corn borer, Ostrinia nubilalis.. Entomol Exp Appl 80: 531–537. [CSA], [CROSSREF]
- Iwuala MOE, Osisiogu IUW, Agbakwuru EOP (1981): Dennettia oil, a potential new insecticide: Test with adult and nymph of Periplanata Americana. and Zonocerus variegate.. J Econ Entomol 74: 249–252. [CSA]
- Marles RJ, Farnsworth NR (1994): Plants as sources of anti-diabetic agent. In: Wagner H, Farnsworth NR, eds., Economic and Medicinal Plants Research, Vol. 6. IL, USA, Academic Press Ltd., pp. 150–187.
- McLaughlin JL, Chang CJ, Smith D (1991): Bench-top bioassays for the discovery of bioactive natural products; an update. In: Atta-Ur-Rahman, ed., Studies in Natural Products Chemistry, Vol. 9. Amsterdam, Elsevier Science Publishers BV, pp. 383–408.
- Meyer BN, Ferrigny NR, Putnam JE, Jacobsen LB, Nichol DE, McLaughlin JL (1982): Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med 45: 31–34. [INFOTRIEVE], [CSA]
- Miller LC, Tainted ML (1957): Estimation of ED50 or LD50 values and their error using log-probit graph paper. Proc Soc Exp Biol Med 57: 261. [CSA]
- Okonkwo EU, Okoye WI (1996): The efficacy of four powders and the essential oils as protectant of cowpea and maize grains against infestation of Callosobrunchus maculatus. (Fabricus) (Coleoptera: Brunchidae) & Strophilus zeamais. (Motschulsky) (Coleoptera: Curculionidae) in Nigeria. Int J Pest Managt 42(2): 143–146. [CSA]
- Schalm OW, Jain NC, Carroll EJ (1975): Veterinary Hematology, 3rd ed. Philadelphia, Lea and Febiger. p. 45.