Abstract
The effect of the aqueous extract of Melia azedarach. Linn. (Meliaceae) against ethylene glycol–induced nephrolithiasis in male Wistar albino rats is summarized in this study. Lithiasis was induced in rats by administering 0.75% ethylene glycol in drinking water for 28 days and was manifested by high urinary calcium, phosphate, oxalate, and low urinary magnesium content. Simultaneous administration of aqueous extract of Melia azedarach. (AEMA; 250 mg/kg body weight) orally for 28 days along with ethylene glycol (0.75%) reduced urinary calcium, oxalate, phosphate, and elevated urinary magnesium level. It also increased the urine volume, thereby reducing the tendency for crystallization. The histopathological studies confirmed the induction of lithiasis as microcrystal deposition was observed in sections of kidney from animals treated with ethylene glycol. This was reduced, however, after treatment with the extract. These observations enable us to conclude that AEMA is effective against ethylene glycol–induced nephrolithiasis.
Introduction
Nephrolithiasis (renal stone formation) is a recurrent disorder predominant in males. The present-day medical management of nephrolithiasis is either costly or not without side effects. Hence, the search for antilithiatic drugs from natural sources has assumed greater importance. Many Indian plants have been quoted to be useful as antilithiatic agents. They are effective with fewer side effects and are also inexpensive. Hence, the Indian plants are constantly being evaluated for possible antilithiatic effects in a systematic manner. One such plant is Melia azedarach. Linn. (Meliaceae), which is used as a CNS depressant (Shrivastava et al., Citation1981), a mild analgesic (Shrivastava & Chauhan, Citation1977), and as an anticancer (Nam & Lee, Citation2000), antiviral (Alche et al., Citation2002), and antifertility agent (Choudhary et al., Citation1990). It is reported to contain chemical constituents including quercetin, rutin, tetranortriterpenoids, catechin, limonoids, new anthraquinone pigments, 4,5 dihydroxy flavone, 6-β. hydroxy-4-stigmasten-3-one, and 6-β. hydroxy-4-campesten-3-one (Mishra & Srivastava, Citation1984). The traditional Indian medicine, Ayurveda, suggests this plant to be antilithiatic, but scientific data supporting this statement is still lacking.
Materials and Methods
Animals
Male albino Wistar rats (150–200 g) were obtained from Chellamuthu Trust (Madurai, India). They were housed in well-ventilated cages (3 to 4 per cage), maintained at 25 ± 2°C under 12-h dark/light cycle. They were fed standard pellet diet and had free access to water. The animals were maintained in these conditions for 1 week before the experimental session. Our institutional animal ethical committee (IAEC) approved this study.
Plant material
Melia azedarach. was collected from the medicinal farm at Kariyapatti and identified by the Department of Botany, The American College (Madurai, India), and a voucher specimen (MAC12252) was deposited in our laboratory. The leaves were shade-dried and coarsely powdered in such a way that the material passed through sieve no. 20 and was retained on sieve no. 40. About 500 g of the dry powder was extracted by hot percolation method using 8 parts of solvent (distilled H2O) for 72 h. The aqueous extract was filtered and concentrated under reduced pressure. The yield was 25 g.
Antilithiatic study
The method of Selvam et al. (Citation2001) was followed to evaluate the antilithiatic effect. The acclimatized animals were divided into four groups of six each designated as G-I, G-II, G-III, and G-IV. The animals of G-I served as the normal control. G-II animals received 0.75% ethylene glycol in drinking water ad libitum. for 28 days and served as the lithiatic control. The G-III group animals received 0.75% ethylene glycol in drinking water ad libitum., along with aqueous extract of Melia azedarach. (AEMA; 250 mg/kg body weight) by oral route for 28 days. G-IV animals received AEMA alone (250 mg/kgbody weight) orally for 28 days.
The 24-h urine samples were collected from rats housed in metabolic cages, on the 7th, 14th, 21st, and 28th days and the volume noted. Urinary calcium (Mustafa & Medeiros, Citation1985), magnesium (Mustafa & Medeiros, Citation1985), phosphorous (Fiske & Subbarow, Citation1925), and oxalate (Hodgkinson & Williams, Citation1972) concentrations were estimated using standard methods. Also, the serum and urinary creatinine levels were estimated. To confirm the incidence of lithiasis, the animals were sacrificed and their kidneys were subjected to histopathological studies.
Histopathological studies
Kidney samples were weighed and fixed rapidly with 10% neutralized formalin (pH 7.4). Sections of kidney fixed in paraffin were prepared and stained with hematoxylin and eosin and observed for pathological changes.
Statistical analysis
The results are expressed as mean±SEM. Statistical analysis was carried out using one-way ANOVA followed by Newman-Keuls multiple range test. Differences below p < 0.05 implied significance.
Results
Urinary data
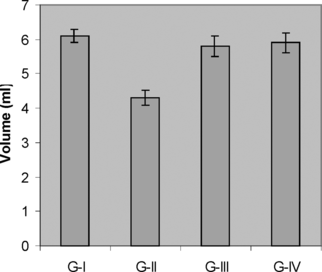
The urinary output of the control and experimental rats on day 28 is shown in . The urinary volume of the control rats (G-I) was 6.1±0.20 ml/day per rat. In ethylene glycol–treated rats (G-II), the urinary output was significantly decreased to 4.3±0.22 ml/day per rat. When compared with G-II, the urinary output of the extract treated group (G-III) was increased (5.8±0.31 ml/day per rat) nearly to that of normal control group, while in the extract alone–treated group (G-IV) the urinary output was found to be 5.90±0.29 ml/day per rat.
Figure 1 Effect of M. azedarach. on urine output during experimental nephrolithiasis. Values are expressed as ml/24 h. Values are mean±SEM for six animals in each group. G-I (control), G-II (EG-treated), G-III (M. azedarach. and EG-treated), G-IV (M. azedarach.–alone treated).
The oxalate excretion was increased significantly on the 14th day in ethylene glycol–treated rats (G-II) compared with the normal control rats (G-I). Maximum oxalate excretion was observed with G-II on the 28th day (3.01±0.30 mg/24 h per rat). However, the oxalate excretion was reduced significantly (1.01±0.10 mg/24 h per rat) in the extract-treated group (G-III), though normal values were not reached. The extract alone–treated rats (G-IV) did not show obvious changes in the ion levels. The results are shown in .
Table 1. Effect of M. azedarach. on oxalate excretion in experimental nephrolithiasis.
Likewise, ethylene glycol treatment increased urinary calcium (4.96±0.67 mg/24 h per rat) and phosphate (33.18±0.15 mg/24 h per rat) excretion significantly in the lithiatic control group (G-II) on the 28th day. However, after treatment with the extract, these values were reduced to 1.75±0.57 mg/24 h per rat and 20.25±4.27 mg/24 h per rat, respectively, in G-III. The results are shown in Tables and .
Table 2. Effect of M. azedarach. on calcium excretion in experimental nephrolithiasis.
Table 3. Effect of M. azedarach. on phosphate excretion in experimental nephrolithiasis.
The magnesium excretion on the 28th day was reduced after treatment with ethylene glycol in G-II (0.53±0.10 mg/24 h per rat). Simultaneous administration of the extract to G-III elevated the reduced magnesium level significantly (2.62±0.35 mg/24 h per rat), when compared with the lithiatic control group (G-II). The results are summarized in .
Table 4. Effect of M. azedarach. on magnesium excretion in experimental nephrolithiasis.
Serum data
The serum creatinine level after ethylene glycol treatment in G-II was 1.70±0.35 mg/dl, which was brought down to 0.60±0.01 mg/dl in G-III after treatment with the extract. Creatinine clearance was also found improved in G-III (0.52±0.04 ml/min). The results are shown in .
Table 5. Effect of M. azedarach. on serum creatinine and creatinine clearance in various groups on day 28.
Histopathological studies
Sections of kidney from animals treated with ethylene glycol showed deposition of microcrystals. There was marked dilation of tubules, tubular damage, and infiltration of inflammatory cells into the interstitial space. However, kidney sections of animals treated with AEMA showed improvement of the above symptoms and reduced crystal deposition as shown in and .
Discussion
Changes in ionic pattern of urine are the major determinant of stone formation. In this study, the ionic pattern was found disturbed by treatment with ethylene glycol. It has been reported that daily oral administration of ethylene glycol for more than 4 weeks resulted in a significant increase in oxalate excretion and that kidneys are the targets for ethylene glycol toxicity (Schladt et al., Citation1998). Ethylene glycol gets oxidized to oxalic acid leading to hyperoxaluria (Underwood et al., Citation1973). Hyperoxaluria is reported to be a more significant risk factor in the pathogenesis of stone formation (Tisselius, Citation1996). Likewise, ethylene glycol administration increased the urinary calcium level. It has been stated that hypercalciuria favors precipitation of calcium oxalate from urine (Lemann et al., Citation1991). Thus, the high oxalate and calcium ion concentration in urine tends to form calcium oxalate crystals. The growth of calcium oxalate crystals is further favored by disturbances in the urinary levels of other ions like magnesium and phosphate. The available literature states that high urinary phosphate level with calcium forms calcium phosphate crystals, which induces further deposition of calcium oxalate on it (Roger et al., Citation1997). In this study, the high urinary phosphate level observed in ethylene glycol–treated rats is likely to have formed calcium phosphate crystals.
Magnesium is a well-known inhibitor of crystallization in urine. This is substantiated by the low level of urinary magnesium observed in stone formers (Rushton & Spector, Citation1982), and also magnesium has been reported to decrease the growth rate of calcium oxalate crystals (Ryall et al., Citation1991; Grases et al., Citation1989). Our study also revealed a similar observation. Thus, ethylene glycol administration induces stone formation by raising urinary calcium, oxalate, and phosphate and by lowering urinary magnesium as noted in G-II.
Our observations showed that AEMA reduced the urinary calcium, oxalate, and phosphate levels. It also raised the urinary magnesium concentration. The increase in urine volume may also minimize the tendency for crystallization. Thus, it is concluded that AEMA has inhibitory potential on ethylene glycol–induced nephrolithiasis.
Because ethylene glycol is reported to be toxic to the kidneys, measuring the serum and urine creatinine levels assessed its influence on kidney function. It was found that kidney function was impaired in the group of animals treated with ethylene glycol alone: however, in the group treated with ethylene glycol and AEMA, the kidney function was found to improve.
Acknowledgment
We thank Prof. M. Nagarajan for his support and encouragement during this work.
References
- Alche LE, Barquero AA, Sanjuan NA, Coto CE (2002): An antiviral principle present in a purified fraction from Melia azedarach. leaf aqueous extract restrains Herpes simplex virus type-1 propagation. Phytother Res 16: 348–352. [INFOTRIEVE], [CSA]
- Choudhary DN, Singh JN, Verma SK, Singh BP (1990): Antifertility effect of leaf extracts of some plants in male rats. Indian J Exp Biol 28: 714–716. [INFOTRIEVE], [CSA]
- Fiske CH, Subbarow Y (1925): The colorimetric determination of phosphorous. J Biol Chem 66: 375–381. [CSA]
- Grases F, Genestar C, Conte A, March P, Costa-Bauza A (1989): Inhibitory effect of pyrophosphate, citrate, magnesium, and chondriotin sulfate in calcium oxalate urolithiasis. Br J Urol 64: 235–237. [INFOTRIEVE], [CSA]
- Hodgkinson A, Williams A (1972): An improved colorimetric procedure for urine oxalate. Clin Chim Acta 36: 127–132. [INFOTRIEVE], [CSA], [CROSSREF]
- Lemann J Jr, Worcestor EM, Gray RW (1991): Hypercalciuria and stones. Am J Kidney Dis 27: 386–391. [CSA]
- Mishra M, Srivastava SK (1984): A new flavone glycoside from Melia azedarach. Linn. Curr Sci 53: 694. [CSA]
- Mustafa MA, Medeiros DM (1985): Proximate composition, mineral content and fatty acids of catfish (Ictalurus punctatus rafinesque.) for different seasons and cooking methods. J Food Sci 50: 585–588. [CSA]
- Nam KA, Lee SK (2000): Evaluation of cytotoxic potential of natural products in cultured human cancer cells. Nat Prod Sci 6: 183–188. [CSA]
- Roger K, Low MD, Stoller ML (1997): Uric acid nephrolithiasis. Urol Clin N Am 24: 135–148. [CSA], [CROSSREF]
- Rushton HG, Spector M (1982): Effects of magnesium deficiency on intratubular calcium oxalate formation and crystalluria in hyperoxaluric rats. J Urol 127: 598–604. [INFOTRIEVE], [CSA]
- Ryall RL, Harnet RM, Marshall VR (1991): The effect of urine pyrophosphate, citrate, magnesium and glycosaminoglycans on the growth and aggregation of calcium oxalate crystals in vitro.. Clin Chim Acta 112: 349–356. [CSA], [CROSSREF]
- Schladt L, Ivens I, Karbe E, Ruhl-Fehlert C, Bomhard E (1998): Sub acute oral toxicity of tetra ethylene glycol and ethylene glycol administered to Wistar rats. Exp Toxicol Pathol 50: 257–265. [INFOTRIEVE], [CSA]
- Selvam R, Kalaiselvi P, Govindaraj A, Balamurugan V, Sathishkumar AS (2001): Effect of Aerva lanata. leaf extract and vediuppu chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol Res 43: 89–93. [INFOTRIEVE], [CSA], [CROSSREF]
- Shrivastava AK, Chauhan CS (1977): Preliminary pharmacological studies of unsaponifiable matter obtained from fixed oil of seeds of Melia azedarach.. Indian Drugs Pharmaceut Ind 12: 39. [CSA]
- Shrivastava AK, Srivastava R, Dixit V (1981): Pharmacological studies on fruits of Melia azedarach.. J Res Ayur Siddha 2: 260. [CSA]
- Tisselius HG (1996): Solution chemistry of super saturation. In: Coe FL, Favus MJ, Pak CYC, Parks JH, Preminger GM, eds., Kidney Stones: Medical and Surgical Management. Philadelphia, Lippincott Raven, p. 33.
- Underwood F, William M, Bennet MD (1973): Ethylene glycol intoxication: Prevention of renal failure by aggressive management. J Am Med Assoc 226: 1453–1454. [CSA], [CROSSREF]


