Abstract
This TDR/WHO project was carried out from 2003 to 2005 in an 0.1-ha biodiversity plot in the Altos de Campana National Park to discover novel active antiparasitic and larvicidal compounds in Panamanian plants. One-hundred-fifty organic plant extracts representing 43 families, 73 genera, and 93 species were tested in a panel of antimalarial (Plasmodium falciparum. W2, chloroquine resistant), antileishmanial (Leishmania mexicana. amastigotes), antitrypanosomal (Trypanosoma cruzi. trypomastigotes), and larvicidal (Aedes aegypti.) screens. Of these 150 plant extracts, two (1.3%) (Talisia nervosa. and Topobea parasitica.) showed significant antimalarial activity (IC50 values < 10 µg/ml), two (1.3%) (Cestrum megalophyllum. and Zanthoxylum acuminatum.) weak antileishmanial activity (IC50 values ranging from 10 to 20 µg/ml), one (0.6%) (Zanthoxylum acuminatum.) weak antitrypanosomal activity (IC50 values ranging from 10 to 20 µg/ml), and one (0.6%) (Piper fimbriulatum.) larvicidal activity (LC100 values < 30 µg/ml). Ethyl gallate (1) and methyl gallate (2) were isolated from stems of Talisia nervosa. by bioassay-guided fractionation. Both (1) and (2) showed weak in vitro. antiplasmodial activity against P. falciparum. (IC50 35.3 µM and IC50 38.0 µM, respectively), but both compounds were less active than chloroquine (IC50 0.088 µM). Moreover, compounds (1) (IC50 33.1 µM) and (2) (IC50 33.6 µM) showed weakly antileishmanial activity (miltefosine: IC50 0.5 µM), but they were not cytotoxic to Vero mammalian cells.
Introduction
The incidence of tropical parasitic diseases is increasing at an alarming rate in the developing countries. Malaria remains as one of the most important diseases of the developing world, affecting 300–500 million people annually and killing 1–3 million people (Fidock et al., Citation2004).
In Panama, malaria has the highest mortality rate (0.1/100,000 inhabitants) among three parasitic diseases (malaria, leishmaniasis and Chagas disease). Recent reports indicate an increase in cases of malaria in provinces of Eastern Panamá and Darien, in the indigenous groups in the Ngöbe-Buglé and Kuna Yala reservations, as well as in the number of cases of dengue in the provinces of Panamá and Chiriquí (Ministerio de Salud de Panamá, Citation2005).
In the chemotherapeutic arsenal for malaria, the two most widely used antimalarial drugs, chloroquine and sulfadoxine-pyrimethamine, are failing at an accelerating rate in most malaria-related morbidity and mortality (Greenwood & Mutabingwa, Citation2002). Today, chloroquine resistance has spread to the vast majority of malaria-endemic areas, rendering this drug increasingly ineffective (Fidock et al., Citation2004). New antimalarial drugs must meet the requirements of rapid efficacy, minimal toxicity, and low cost.
The discovery of artemisinin from Artemisia annua. more than 30 years ago provided a completely new antimalarial structural prototype (Vennerstrom et al., Citation2004). After this finding, the chemotherapy of malaria has benefited greatly from the semisynthetic artemisinins, artemether and atesuante, as they rapidly reduce parasite burden, have good therapeutic indices, and provide successful treatment outcomes. However, as a drug class, the artemisinins pose some chemical, biopharmaceutical, and therapeutic problems that limit their potential.
The chemotherapy of human leishmaniasis and Chagas disease is currently precarious (Croft et al., Citation1997). Arsenical and antimonial drugs for the treatments of leishmaniasis were among the first synthetic drugs for infectious diseases. It is remarkable that organic derivatives of these organometallic drugs (sodium stilbogluconate, meglumine antimonite, pentamidine) used in the first decade of the twentieth century remain today the drugs of choice. The need for new treatments has become more urgent due to the spread of resistance to antimonials and the emergence of visceral leishmaniasis.
In contrast, no drug was available to treat Chagas disease until the 1970s when two nitroheterocyclic drugs (benznidazole and nifurtimox) were introduced. To combat malaria, leishmaniasis, and Chagas disease, new drugs are desperately needed in the developing world.
Regarding dengue, vector control in the prevention of dengue transmission is the most important approach nowadays because there are no vaccines and drugs available (Chadee et al., Citation2005). Therefore, there is an urgent need to understand better the environmental factors that contribute to the proliferation of the disease vector Aedes aegypti..
A constant valuable source of new drugs to treat these parasitic diseases is the plant kingdom. Therefore, Panamanian flora has been selected to search for lead compounds. Panamanian flora comprises 9520 vascular plants, of which 12.0% are endemic (Correa et al., Citation2004). Panamá has a higher density (no. of species/10,000 km2) (0.011) of species of angiosperms than Brazil (0.006) (Correa et al., Citation2004).
The current work aims at screening organic plant extracts for antileishmanial, antimalarial, antitrypanosomal, and larvicidal activities and subsequently to isolate and characterize bioactive molecules.
Materials and Methods
Selection of plants
As a criterion for plant selection of this study, the biodiversity-plot approach was used because it is the best indication of diversity of plant species in a neotropical forest (Berry, Citation2003). It is also known that high diversity of plants in a particular flora implies high diversity of chemical structures (Cordell, Citation1995). The plot of the study was established at Altos de Campana National Park (PNAdC), which is located on the Pacific coast, 60 km southeastern of Panamá City. This park has an extension of 4816 ha covered by tropical rain forest, tropical premontane and tropical montane forests. The selected plot at PNAdC measured 0.1 h (10 m × 100 m). At the plot, 580 individuals were registered (plants with ≥1 cm of diameter at breast height), which represented 74 families, 160 genera, and 243 species.
Plant material
Plants were collected from an 0.1-ha plot at the Altos de Campana National Park, Buena Vista, Chame, located at N08° 41′49.26″ W079° 57′47.46″, and from an area outside of the plot within a 50-mile radius and from nearby provinces when there was only one individual of a species in the plot by the botanists Alex Espinosa, Carlos Guerra, Libardo Martínez, and Carmen Galdames. Their taxonomic identity was established by Mireya Correa, and the voucher specimens are deposited at the herbarium of the University of Panamá (PMA). Detailed information about specimens can be obtained from the corresponding author upon request.
Extraction and isolation
Preparation of extracts
Different plant parts were macerated with one of the organic solvents (80% EtOH, MeOH, or CHCl3) for extraction. The plant extracts were filtered and concentrated in vacuo. at <40°C in a rotary evaporator and stored at −80°C until further use. Each active plant was initially fractionated using the liquid-liquid partition method of Kupchan (Hussein et al., Citation2003) followed by various types of column chromatography. The bioassay-guided isolation was conducted for the active extracts using the bioassays described later.
Isolation of bioactive compounds
Air-dried stems (402 g) of Talisia nervosa. were cut into small pieces and macerated at room temperature for 24 h in 80% EtOH (3 days). After filtration, the solvent was removed in a rotary evaporator under reduced pressure to give 15.0 g of extract. It was then suspended in water and successively partitionated with CHCl3. Then, the aqueous phase was partitionated with EtOAc. Finally, this procedure gave CHCl3 fraction (0.2 g), EtOAc fraction (2.4 g), and H2O fraction (12.4 g). The EtOAc fraction (500 mg) was subjected to low-pressure liquid chromatography (RP-18) and on elution with a gradient of MeOH-H2O (8:2 → 100:0) resulted in 10 fractions of 20 ml each (TN-1 to TN-10). TN-6, which was eluted with MeOH-H2O (4:6), gave 5 mg of pure compound (1), and TN-8 (17 mg), eluted with MeOH-H2O (1:1), was further purified by precipitation to give 15 mg of compound (2).
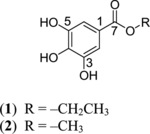
Ethyl gallate. (.1.).: 1H NMR (CD3OD, 300 MHz) δ 7.04 (1H, s, H-2, H-6), 4.26 (2H, q, H-8), 1.34 (1H, t, CH3-9). 13C NMR (CD3OD, 75 MHz) δ 169.0 (C-7), 146.8 (C-3, C-5), 140.3 (C-4), 122.7 (C-1), 110.5 (C-2, C-6), 62.0 (C-8), 15.0 (C-9). HRTOFMS m./z. [M + H] 199.0605 C9H11O5.
Methyl gallate. (.2.).: 1H NMR (CD3OD, 300 MHz) δ 7.04 (1H, s, H-2, H-6), 3.81 (3H, s, CH3-8). 13C NMR (CD3OD, 75 MHz) δ 169.9 (C-7), 147.4 (C-3, C-5), 140.7 (C-4), 122.4 (C-1), 110.9 (C-2, C-6), 53.2 (C-8). HRTOFMS: [M + H] m./z. 185.0472 C8H9O5.
Biological assays
Antiplasmodial assay
Antiplasmodial activity was determined in a chloroquine-resistant Plasmodium falciparum. clone (W2 Indochina) using a novel microfluorimetric assay to measure the inhibition of the parasite growth based on the detection of the parasitic DNA by intercalation with PicoGreen (Corbett et al., Citation2004). The IC50 values were calculated from relative fluorescence units as compared with untreated controls. The parasites were maintained at 2% hematocrit in flat-bottom flasks (75 ml) in human red blood cells from O-positive blood type donor with RPMI 1640 medium (Gibco BRL Gaithersburg, MD, USA) supplemented with 10% O-positive human serum. Chloroquine was used as a standard antimalarial agent.
Antileishmanial assay
The study used a fluorimetric Leishmania mexicana. (WHO-MOHM/B2/82/BELZ) amastigote assay adapted from a novel antimalarial micromethod by Corbett et al. (Citation2004) to measure inhibition of parasite growth based on the detection of parasitic DNA by intercalation with PicoGreen. Briefly, 25,000 amastigotes from axenic culture (Bates, Citation1993) were coincubated with the test substances at 32°C in a CO2-free atmosphere during 72 h. The relative fluorescence units (RFU) were quantified with a fluorescence microplate reader (FLx800; Bio-Tek Instruments, Inc., Winooski, VT, USA) at 485/20 nm excitation and 528/20 nm emission filters. Amphotericin B was used as a standard antileishmanial agent.
Axenic antileishmanial activity
Amastigotes of Leishmania donovani. strain MHOM/ET/67/L82 were grown in axenic culture at 37°C in SM medium (Cunningham, Citation1977) at pH 5.4 supplemented with 10% heat-inactivated fetal bovine serum under an atmosphere of 5% CO2 in air. Culture medium with 105 amastigotes from axenic culture (100 µl) with or without a serial drug dilution were seeded in 96-well microtiter plates. Seven three-fold dilutions were used covering a range from 30 to 0.041 µg/ml. After 72 h of incubation, the plates were inspected under an inverted microscope to ensure growth of the controls and sterile conditions. Alamar blue (10 µl of 12.5 mg resazurin dissolved in 100 ml distilled water) was then added to each well and the plates incubated for another 2 h. Then the plates were read with a Spectramax Gemini XS microplate fluorometer (Molecular Devices Corporation, Sunnyvale, CA, USA) using an excitation wavelength of 536 nm and an emission wavelength of 588 nm. Data were analyzed using the software Softmax Pro (Molecular Devices Corporation). Decrease of fluorescence (= inhibition) was expressed as percentage of the fluorescence of control cultures and plotted against the drug concentrations. The IC50 values were calculated from the sigmoidal inhibition curves.
Antitrypanosomal assay
The recombinant Tulahuen clone C4 of Trypanosoma cruzi., which expresses β-galactosidase (βGal) as a reporter enzyme, was used in the assay (Buckner et al., Citation1996). The method was based on the growth inhibition effect of the test samples on trypomastigote, the intracellular form of the parasite. The resulting color from the cleavage of chlorophenol red-β-D-galactopyranoside (CPRG) by β-Gal, expressed by the parasite, was measured at 570 nm. The inhibition concentration of 50% (IC50) as compared with the untreated control was calculated from the optical density values. Assays were conducted at 37°C under an atmosphere of 5% CO2/95% air mixture. Nifurtimox was tested as a standard antitrypanosomal agent.
Larvicidal assay
The larvicidal activity was determined on larvae of Aedes aegypti. in a 96-well plate according to the method of Ceplenau (1993), modified by Solís et al. (Citation1996). Tetrametrin was used as a standard larvicidal agent.
Cytotoxicity assay
Vero cells (Cercopithecus aethiops., African green monkey) adhering to 96-well plates were used to evaluate the toxicity of the assayed compounds after 72 h coincubation at 37°C in a CO2 atmosphere. Cell viability was evaluated on the basis of the color produced by reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma St. Louis, MD, USA) by the mitochondrial dehydrogenase by the alive cells (Torres-Mendoza et al., Citation2004). Optical densities were measured in a Benchmark plate reader (Bio-RAD, Hercules, CA, USA) employing a 570-nm test wavelength filter and a 650-nm reference filter.
Results and Discussion
Ninety-three plants out of 243 were selected on the basis of biodiversity-plot approach (Mayo & Correa, Citation1997) at the Altos de Campana National Park and literature search to discover novel and active compounds in Panamanian plants. The bioassay-guided isolation and structure elucidation of compounds from active plants are presented.
Among the selected plants from the plot, most of them belong to the more representative plant families of Panamá: Acanthaceae, Euphorbiaceae, Fabaceae, Melastomataceae, Myrsinaceae, Piperaceae, Rubiaceae, and Solanaceae. Moreover, genera with the highest number of sampled species were Piper. and Psychotria.. The most common species in the plot was Talisia nervosa..
Biological evaluation of extracts
Larvicidal and in vitro. antiparasitic activities of the extracts are summarized in . In the larvicidal assay, an extract is considered active when the LC100 is <30 µg/ml (Solís et al., Citation1996), while in the in vitro. antiparasitic screening, extracts with IC50 < 10 µg/ml are considered highly active and weakly active with IC50 ranging from 10 to 20 µg/ml (Vonthon-Sénécheau et al., Citation2003; Hoet et al., Citation2004; Mbwanbo et al., Citation2004; Weniger et al., Citation2004; Özipek et al., Citation2005; Tasdemir et al., Citation2005).
Table 1.. Larvicidal and antiparasitic activities of plant extracts.
In vitro antiparasitic screening
One-hundred-fifty plant extracts were screened, two extracts (1.3%) showed significant activity against Plasmodium falciparum. (W2) at IC50 < 10 µg/ml, and seven (4.7%) extracts were weakly active against P. falciparum. (W2) at IC50 ranging from 10 to 20 µg/ml. Therefore, 141 (94%) extracts were inactive against the same parasite.
Moreover, two extracts (1.3%) showed weak activity against Leishmania mexicana. and 148 (98.6%) extracts were inactive against this parasite. One (0.7%) extract showed weak activity against Trypanosoma cruzi.. Consequently, 149 (99.3%) extracts were inactive against this parasite.
The most active extract in each of these assays was Talisia nervosa. (IC50 6.0 µg/ml, leaves; IC50 11.0 µg/ml, stems; P. falciparum. (W2), Cestrum megalophyllum. (leaves) and Zanthoxylum acuminatum. (stems) (IC50 17.0 µg/ml; L. mexicana.), Zanthoxylum acuminatum. (stems) (IC50 17.0 µg/ml; T. Cruzi.), and Piper fimbriulatum. (LC100 6.25 µg/ml; A. aegypti.).
Certain facts such as the presence of syncarpanide and decarine, antiplasmodial alkaloids (IC50 < 10 µg/ml) from Zanthoxylum syncarpum. (Ross et al., Citation2004), and the report on antiplasmodial activity of Zanthoxylum chalybeum. (IC50 < 10 µg/ml) (Gessler et al., Citation1994) may suggest from a chemotaxonomic point of view the antiplasmodial activity of Zanthoxylum acuminatum. reported in this study.
There is no specific report on trypanocidal activity of Z. acuminatum., but a lignan, called (−)-methylpluviatolide, has been isolated from leaves of Z. naranjillo., with in vitro. trypanocidal activity (Bastos et al., Citation1999).
In addition to Garcinia madruno., various species of Garcinia. have displayed antiplasmodial activity: G. kola. (seed and stembark, CH2Cl2; IC50 6.0 µg/ml) (Tona et al., Citation1999), G. livingstonei. (rootbark, MeOH; IC50 10.0 µg/ml P. falciparum. W2), and G. macgregorii. (roots, MeOH; IC50 10.0 µg/ml P. falciparum. W2) (Horgen et al., Citation2001). In terms of chemistry, antiplasmodial xanthones have been found in Garcinia vieillardi. (Hay et al., Citation2004), Garcinia dulcis. (Likhitwitayawuid et al., Citation1998a) and Garcinia cowa. (Likhitwitayawuid et al., Citation1998b). Moderate antiplasmodial activity of Euterpe precatoria. can be explained by the presence of 8-5′ linked lignan dehydrodiconiferyl dibenzoate (Jensen et al., Citation2002). Few reports on the chemistry of Cestrum. species indicate the presence of cytotoxic steroidal saponins from leaves of C. nocturnum. (Mimaki et al., Citation2001) and C. sendtenerianum. (Haraguchi et al., Citation2000), but there is no report on antileishmanial activity of C. megallophylum..
Diogenone, a steroid with in vitro. antiplasmodial activity, has been isolated from Solanum nudum. (Pabon et al., Citation2002). This compound did not show in vitro. mutagenicity or clastogenic effects (Pabon et al., Citation2003; Alvarez et al., Citation2004). The in vivo. antiplasmodial activity was demonstrated in mice infected with P. berghei. (Echeverri et al., Citation2001). Another species, Solanum erianthum. (aqueous leaf extract), showed activity on albino Swiss mice infected with P. berghei. (Makinde et al., Citation1987). These published activities in different species of the genus Solanum. may support the antiplasmodial activity of S. lanceifolium..
During the development of this project, 7-epi.-sesartemin, an antiplasmodial lignan, was isolated from the leaves of P. fimbriulatum. (Solis et al., Citation2005). Moreover, antiplasmodial compounds sarmentine and 1-piperettyl pyrrolidine were isolated from Piper sarmentosum. (Rukachaisirikul et al., Citation2004), and chabamide was isolated from Piper chaba. (Rukachaisirikul et al., Citation2002).
Other reports on biological activities against T. cruzi. of species of Piper. indicated that 3-farnesyl-2-hydroxy benzoic acid isolated from P. multiplinervium. displayed a weak trypanocidal activity (Rüegg et al., Citation2006).
Currently, no data are available regarding the in vitro. antiplasmodial, antileishmanial, and antitrypanosomal activity for the species or genera of the following plants: Talisia nervosa., Topobea parasitica., Macrocnemum roseum., Gordonia fruticosa., and Cestrum megallophylum..
Larvicidal screening
Of 150 plant extracts, 149 were considered inactive against A. aegypti. with LC100 < 30 µg/ml. Only the chloroform extract of Piper fimbriulatum. (leaves) showed activity at LC100 6.25 µg/ml. The compound responsible for the significant larvicidal activity against A. aegypti. was 7′-epi.-sesartemin (Solis et al., Citation2005).
Bioactivity of isolated compounds
Seven bioactive compounds (two new and five known) were isolated from three plants: Talisia nervosa. (two gallates), Piper fimbriulatum. (three lignans and one flavonoid) (Solís et al., Citation2005), and Piper multiplinervium. (one benzoic acid derivative) (Rüegg et al., 2005).
Ethyl gallate (1) and methyl gallate (2) were isolated from stems of Talisia nervosa.. Their structures (1 and 2, ) were confirmed by comparison of 1H and 13C NMR and MS spectra with those described in the literature (Kane et al., Citation1988; Salmon & Walls, Citation1966). Ethyl gallate (1) was isolated for the first time from seeds of Rhynchosia phaseoloides. (Sw.) DC. (Fabaceae) (Salmon & Walls, Citation1966), and methyl gallate (2) was previously found in Sapium sebiferum. (L.) Roxb. (Euphorbiaceae).
Compound 1 (IC50 35.3 µM) showed a similar very weak antiplasmodial activity as compound 2 (IC50 38.0 µM). Chloroquine was used as a standard antiplasmodial compound, showing an IC50 of 0.088 µM. They both did not display cytotoxicity against Vero mammalian cells (IC50 > 10 µg/ml). The SI (selective index) of compounds 1 and 2 was defined as the ratio of the IC50 value on the mammalian cells to the IC50 value on Plasmodium falciparum. W2. Compounds 1 (SI > 5.5) and 2 (SI > 41.5) demonstrated high selectivity for P. falciparum. W2.
In the axenic Leishmania donovani. screening, compounds 1 and 2 showed weak activity with IC50 values of 33.1 and 33.6 µM, respectively. Additionally, 3-farnesyl-2-hydroxy benzoic acid isolated from P. multiplinervium. (Rüegg et al., 2005) was also tested in this screening. It displayed a moderate activity with an IC50 value of 9.6 µM. Miltefosine was used as reference drug (IC50 0.5 µM). The three tested compounds were not toxic to myoblast (L6) cells, showing IC50 values of 48.6, 44.5, and 84.4 µg/ml, respectively. The reference drug was podophyllotoxin (IC50 0.006 µg/ml). This is the first report of a weak antiplasmodial activity of ethyl gallate and of methyl gallate and of a moderate antileishmanial activity of ethyl gallate, methyl gallate, and 3-farnesyl-2-hydroxy benzoic acid.
Ethyl gallate and methyl gallate have been reported to possess other biological activities such as antioxidant effect on free-radical scavenging (Op de Beck et al., Citation2003) and relaxant effect in guinea-pig trachea in vitro. by activation of large conductance Ca2+ activated K+channels (Paulino et al., Citation1999). The only report on the chemistry of the genus Talisia. is about the isolation of isolectins from T. escualenta. (Freire et al., Citation2001). There is a report of potent inhibition of platelet aggregation by collagen and by arachidonic acid of methyl gallate (Lim et al., Citation2004). Moreover, methyl gallate displayed a potent activity against herpes simplex in vitro. (Kane et al., Citation1988).
Thus, the results of the antiparasitic and larvicidal screening of plants from a plot of Altos de Campana National Park open avenues to understand better the diversity of the Panamanian tropical forest and its potential as a source of active antiparasitic and larvicidal compounds.
Acknowledgments
This work was supported by the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR grant ID A20726 to A.I.C.), the Organization of American States (OAS), and Foundation Natura. The authors are thankful to J. Gonzalez, L. Ureña, L. Abrego, and Z. Capitan Barrios (INDICASAT, Panama) for antiparasitic testing and to Dr. Todd Williams (University of Kansas, USA) for the MS measurements. Thanks are also due to the National Environmental Authority for granting permission to collect plants in a national park.
References
- Alvarez G, Pabon A, Carmona J, Blair S (2004): Evaluation of clastogenic potential of the antimalarial plant Solanum nudum.. Phytother Res 18: 845–848.
- Bastos JK, Albuquerque S, Silva ML (1999): Evaluation of the trypanocidal activity of lignans isolated from the leaves of Zanthoxylum naranjillo.. Planta Med 65: 541–544.
- Bates PA (1993): Axenic culture of Leishmania. amastigotes. Parasitol Today 9: 143–146.
- Berry PE (2003): Diversidad y endemismo en los bosques neotropicales de bajura. In: Guariguata MR, Kattan GH, eds., Ecología y Conservación de Bosques Neotropicales, 1st ed., Ediciones LUR, Cartago, pp. 83–96.
- Buckner FS, Verlinde CLMJ, La Flamme AC, Van Boris WC (1996): Efficient technique for screening drugs for activity against Trypanosoma cruzi. using parasites expressing β-galactosidase. Antimicrob Agents Chemother 40: 2592–2597.
- Cepleanu F (1993): Validation and applications of three bench-top bioassays for screening of crude extracts and subsequent activity-guide isolation. Ph.D. Thesis, University of Lausanne, Lausanne, Switzerland, pp. 102–119.
- Chadee DD, Williams FL, Kitron UD (2005): Impact of vector control on a dengue fever outbreak in Trinidad, West Indies, in 1998. Trop Med Int Health 10: 748–754.
- Corbett Y, Herrera L, Cubilla L, Capson TL, Coley PD, Kursar TA, Romero LI, Ortega-Barría E (2004): A novel DNA-based microfluorimetric method to evaluate antimalarial drug activity. Am J Trop Med Hyg 70: 119–124.
- Cordell GA (1995): Changing strategies in natural products chemistry. Phytochemistry 40: 1585–1612.
- Correa M, Galdames C, Stapf M (2004): Catálogo de Plantas Vasculares de Panamá. Panamá, Editora Novo Art, S.A., p. 600.
- Croft SL, Urbina JA, Brun R (1997): Chemotherapy of human leishmaniasis and trypanosomiasis. In: Hide G, Mottram JC, Coombs GH, Holmes PH, eds., Trypanosomiasis and Leishmaniasis. Biology and Control. Oxford, CAB International, pp. 245–257.
- Cunningham I (1977): New culture medium for maintenance of tsetse tissues and growth of trypanosomatids. J Protozool 24: 325–329.
- Echeverri M, Blair S, Carmona J, Perez P (2001): Effect of Solanum nodum. extracts on the liver of mice infected with Plasmodium berghei.. Am J Chin Med 29:477–484.
- Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S (2004): Antimalarial drug discovery: Efficacy models for compound screening. Nat Rev 3: 509–520.
- Freire MDGM, Machado OLT, Smolka MB, Marangoni S, Novello JC, Macedo MLR (2001): Isolation and characterization of isolectins from Talisia esculenta. seeds. J Protein Chem 20: 495–500.
- Gessler MC, Nkunya MH, Mwasumbi LB, Heinrich M, Tanner M (1994): Screening Tanzanian medicinal plants for antimalarial activity. Acta Trop 56: 65–77.
- Greenwood B, Mutabingwa T (2002): Malaria in 2002. Nature 415: 670–672.
- Haraguchi M, Mimaki Y, Motidome M, Morita H, Takeya K, Itokawa H, Yokosuka A, Sashida Y (2000): Steroidal saponins from the leaves of Cestrum sendtenerianum.. Phytochemistry 55: 715–20.
- Hay AE, Helesbeux JJ, Duval O, Labadied M, Grellier P, Richmme P (2004): Antimalarial xanthones from Calophyllum caledonicum. and Garcinia vieillardii.. Life Sci 75: 3077–3085.
- Hoet S, Opperdoes F, Brun R, Adjakidjé V, Quetin-Leclercq J (2004): In vitro. antitrypanosomal activity of ethnopharmacologically selected Beninese plants. J Ethnopharmacol 91: 37–42.
- Horgen FD, Edrada RA, De los Reyes G, Agcaoili F, Madulid DA, Wongpanich V, Angerhofer CK, Pezzuto JM, Soejarto DD, Farnsworth NR (2001): Biological screening of rain forest plot trees from Palawan Island (Philippines). Phytomedicine 81: 71–81.
- Hussein AA, Bozzi B, Correa M, Capson TL, Kursar TA, Coley P, Solis PN, Gupta MP (2003): Bioactive constituents from three Vismia. species. J Nat Prod 66: 858–860.
- Jensen JF, Kvist LP, Christensen SB (2002): An antiplasmodial lignan from Euterpe precatoria.. J Nat Prod 65: 1915–1917.
- Kane CJ, Menna JH, Yeh YC (1988): Methyl gallate, methyl-3,4,5-trihydroxy benzoate, is a potent and highly specific inhibitor of Herpes simplex. virus in vitro.. I. Purification and characterization of methyl gallate from Sapium sebiferum.. Biosci Rep 8: 85–94.
- Likhitwitayawuid K, Chanmahasathien W, Ruangrungsi N, Krungkrai J (1998a): Xanthones with antimalarial activity from Garcinia dulcis.. Planta Med 64: 281–282.
- Likhitwitayawuid K, Phadungcharoen T, Krungkrai J (1998b): Antimalarial xanthones from Garcinia cowa.. Planta Med 64: 70–72.
- Lim MY, Park YH, Son DJ, Kim MK, Lee HS (2004): Antiplatelet activity of gallic acid and methyl gallate. Food Sci and Biotechnol 13: 806–809.
- Makinde JM, Obih PO, Jimoh AA (1987): Effect of Solanum erianthum. aqueous leaf extract on Plasmodium berghei. in mice. Afr J Med Sci 16: 193–196.
- Mayo E, Correa MD (1997): Antecedentes de datos biológicos e inventario biológico de la Cuenca del Canal. Scientia 3: 1–455.
- Mbwambo ZH, Apers S, Moshi MJ, Kapingu MC, Van Miert S, Claeys M, Brun R, Cos P, Pieters L, Vlietinck A (2004): Anthranoid compounds with antiprotozoal activity of Vismia orientalis.. Planta Med 70: 691–782.
- Mimaki Y, Watanabe K, Ando Y, Sakuma C, Sashida Y, Furuya S, Sakagami H (2001): Flavonol glycosides and steroidal saponins from the leaves of Cestrum nocturnum. and their cytotoxicity. J Nat Prod 64: 17–22.
- Ministerio de Salud de Panamá (2005): Informe sobre casos, defunciones y tasas por 100,000 habitantes de enfermedades de la Dirección General de Salud Pública: Malaria, Leishmaniasis, Enfermedad de Chagas y Dengue. Panamá, Ministerio de Salud de Panamá.
- Op de Beck P, Cartier G, David B, Dijoux-Franca MG, Mariotte AM (2003): Antioxidant flavonoids and phenolic acids from leaves of Leea guineense. G Don (Leeaceae). Phytother Res 17: 345–347.
- Özipek M, Dömez AA, Calis I, Brun R, Rüedi P, Tasdemir D (2005): Leishmanicidal cycloartane-type triterpene glucosides from Astragalus oleifolius.. Phytochemistry 66: 1168–1173.
- Pabon A, Blair S, Carmona J, Zuleta M, Saez J (2003): Evaluation of the mutagenicity of antimalarial products isolated from Solanum nudum. (Solanaceae). Pharmazie 58: 263–267.
- Pabon A, Carmona J, Maestre A, Camargo M, Blair S (2002): Inhibition of P. falciparum. by steroids isolated from Solanum nudum.. Phytother Res 16: 59–62.
- Paulino N, Pizollatti MG, Yunes RA, Filho VC, Creczynski-Pasa TB, Calixto JB (1999): The mechanisms underlying the relaxant effect of methyl and ethyl gallates in the guinea pig trachea in vitro.: Contribution of potassium channels. Naunyn-Schmiedebergs Arch Pharmacol 360: 331–336.
- Ross SA, Sultana GN, Burandt CL, ElSohly MA, Marais JP & Ferreira D (2004): Syncarpamide, a new antiplasmodial (+)-norepinephrine derivative from Zanthoxylum syncarpum.. J Nat Prod 67: 88–90.
- Rukachaisirikul T, Prabpai S, Champung P, Suksamram A (2002): Chabamide, a novel piperine dimer from stems of Piper chaba.. Planta Med 68: 853–855.
- Rukachaisirikul T, Siriwattanakit P, Sukcharoenphol K, Wongvein C, Ruttanaweang P, Wongwattanavuch P, Suksamrarn A (2004): Chemical constituents and bioactivity of Piper sarmentosum.. J Ethnopharmacol 93: 173–176.
- Rüegg T, Calderón AI, Queiroz EF, Solís PN, Marston A, Rivas F, Ortega-Barría, E, Hostettmann K, Gupta MP (2006): 3-Farnesyl-2-hydroxybenzoic acid is a new anti- H. pylori. compound from Piper multiplinervium.. J Ethnopharmacol 103: 461–467.
- Salmon M, Walls F (1966): Isolation of ethyl gallate from Rhynchosia phaseoloides.. Bol Inst Quim Univ N 18: 34–37.
- Solís PN, Olmedo DA, Avila JE, Correa MD, Gupta MP (1996): Un nuevo ensayo larvicida para detectar plantas con actividad anti-Aedes aegypti.. XV Congreso Científico Nacional, Universidad de Panamá, República de Panamá, October 21–25, Poster No. 18.
- Solís PN, Olmedo D, Nakamura N, Calderón AI, Hattori M, Gupta MP (2005): A new larvicidal lignan from Piper fimbriulatum.. Pharm Biol 43: 378–381.
- Tasdemir D, Güner ND, Perozzo R, Brun R, Domes AA, Calis I, Rüedi P (2005): Anti-protozoal and plasmodial FabI enzyme inhibiting metabolites of Scrophularia lepidota. roots. Phytochemistry 66: 355–362.
- Tona L, Ngimbi NP, Tsakala M, Mesia K, Cimanga K, Apers S, De Bruyne T, Pieters L, Totte J, Vlietinck AJ (1999): Antimalarial activity of 20 crude extracts from nine African medicinal plants used in Kinshasa, Congo. J Ethnopharmacol 68: 193–203.
- Torres-Mendoza D, Ureña Gonzalez LD, Ortega-Barria E, Coley PD, Kursar TA, Capson TL, McPhail K, Cubilla-Rios L (2004): Novel cassane and cleistanthane diterpenes from Myrospermum frutescens.: Absolute stereochemistry of the cassane diterpene series. J Nat Prod 67: 1711–1715.
- Vennerstrom JL, Arbe-Barnes S, Brun R, Charman SA, Chiu FCK, Chollet J, Dong Y, Dorn A, Hunziker D, Matile H, Mcintosh K, Padmanilayam M, Santo Tomas J, Scheurer C, Scorneaux B, Tang Y, Urwyler H, Wittlin S, Charman WN (2004): Identification of an antimalarial synthetic trioxolane drug development candidate. Nature 430: 900–904.
- Vonthon-Sénécheau C, Weniger B, Ouattara M, Trai Bi F, Kamenan A, Lobstein A, Brun R, Anton R (2003): In vitro. antiplasmodial activity and cytotoxicity of ethnobotanically selected Ivorian plants. J Ethnopharmacol 87: 221–225.
- Weniger B, Latifou L, Vonthron-Sénécheau C, Adjobimey T, Gbenou J, Moudachirou M, Brun R, Anton R, Sanni A (2004): Evaluation of ethnobotanically selected Benin medicinal plants for their in vitro. antiplasmodial activity. J Ethnopharmacol 90: 279–284.