Abstract
The flavonoid baicalin, isolated from the dried root of Scutellaria baicalensis. G. (Labiatae), is widely used in traditional Chinese herbal medicine. In the present study, baicalin, at doses of 20, 40, and 80 mg/kg (p.o.), reduced immobility time in tail suspension test (TST) and the forced swimming test (FST) in mice. Baicalin also decreased immobility time at 12.5, 25, and 50 mg/kg (p.o.) in FST in rats. Furthermore, baicalin (25 mg/kg), as well as fluoxetine (FLU; 20 mg/kg), showed a significant recovery in sucrose intake compared with the vehicle-treated stressed animals for 5 weeks treatment in a chronic mild stress (CMS) model in rats. The effect of baicalin at the dose of 25 mg was as potent as that of reference antidepressant FLU (20 mg/kg) in the CMS model. With the monoamine oxidase (MAO A and B) assay, oral administration of baicalin at the doses of 12.5, 25, and 50 mg/kg significantly inhibited MAO A activity in a dose-dependent manner in rats. However, only baicalin at the doses of 25 and 50 mg/kg markedly inhibited MAO B activity. Neither baicalin nor FLU, at the doses tested, produced a significant effect on locomotor activity in mice. These results suggest that baicalin had a specific antidepressant-like effect in vivo.. The antidepressant activity of baicalin may be mediated in part through MAO A and B inhibition in rat brain.
Introduction
Major depression is one of the most prevalent psychiatric disorders in both the Eastern and Western areas of the world and is characterized by a significant change in mood that is protracted and severely debilitating (Lin et al., Citation2002). Antidepressants have been used in clinics for several decades. Although all of them possess certain therapeutic actions, most of them inevitably have some serious adverse effects such as cardiovascular disease, the narrow scope of remedial spectrum, and a short half-life. Therefore, there is an urgent need for research and development of more effective antidepressants without any (or with lower) adverse effects. Combined treatments for some chronic diseases, including affective disorders, with both Western and traditional Chinese medicine, are not unusual in China.
In China, the dried root of Scutellaria baicalensis. G. (Labiatae) (common name, “huang qin”) has been widely employed in traditional Chinese medicine for many centuries. Scutellaria baicalensis. is one of the major constituents in “chaihu-jia-longgu-muli-tnag,” a traditional Chinese formula, which is widely used for the treatment of depression in folk medicine and also has been shown to have a significant antidepressant-like activity in animal models (Qu et al., Citation2003). Several major flavonoids, including baicalein, baicalin (baicalein 7-D-β.-glucuronate), oroxylin, wogonin, and β.-sitosterol, have been isolated from this plant. As the major constituent of Scutellaria baicalensis., baicalin possesses many therapeutic properties including antithrombotic (Kubo et al., Citation1985; Kimura et al., Citation1997), antibacterial (Huang, Citation1999), antiviral (Nagai et al., Citation1995; Kitamura et al., Citation1998), antipyretic, antitoxin, antioxidant (Gao et al., Citation1995; Shi et al., Citation1995; Gabrielska et al., Citation1997), antitumor (Okita et al., Citation1993; Huang et al., Citation1994; Motoo & Sawabu, Citation1994; Yano et al., Citation1994; Matsuzaki et al., Citation1996; Fukutake et al., Citation1998; Kato et al., Citation1998), and anti-inflammatory (Lin & Shieh, Citation1996) effects, inhibition of prostaglandin E2 production (Kyo et al.,Citation1998; Nakahata et al.,Citation1998), reduction of high blood pressure, and relaxation of arterial smooth muscle cells (Chen et al., Citation1999). This drug has been shown to be relatively nontoxic when given orally, but intramuscular injection can cause fever and muscle aches (Huang, Citation1999). However, information regarding the antidepressant activity of baicalin is lacking. In the present study, we investigated the possible antidepressant-like effect of baicalin in three animal models: the tail suspension test (TST), the forced swimming test (FST), and the chronic mild stress (CMS) model, as well as monoamine oxidase (MAO) activity in rat whole brain.
Materials and Methods
Extraction of baicalin
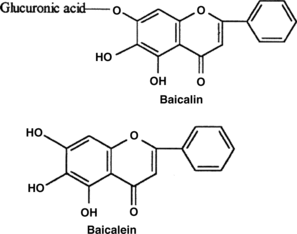
Baicalin (MW 446.38) was extracted from the ground roots of S. baicalensis. with hexane, acetone, and finally with methanol as described previously (Chen et al., Citation2000). In brief, the ground root powder of huang qin was extracted with hexane and acetone. About 3 g of acetone extract was dissolved in 180 ml methanol and then placed overnight in a freezer (−20°C). The yellow precipitate obtained was filtered, redissolved in methanol, and placed again overnight at −20°C. This procedure of crystallization was performed four more times in methanol at −4°C filtered air. The final yellow crystals (about 240 mg) obtained were pure baicalin, as shown by thin-layer chromatography and NMR analysis. It had a melting point of 222–224°C, and λmax at 279 and 314 nm in methanol. The chemical structures of baicalin and baicalein (MW 270.25) are shown in .
Reagents and chemicals
Fluoxetine (FLU; purity 99%) was bought from Sigma (St. Louis, MO, USA). 5-Hydroxytryptamine, benzylamine, and β.-phenylethylamine were purchased from Sigma. All other reagents used in the study were of analytical grade.
Animals
Male Kunming mice weighing 18–22 g and male Wistar rats weighing 220–260 g were used for TST and FST. Mice were randomly assigned to seven equal groups (vehicle control, 10 mg/kg FLU or 10, 20, 40, 80, or 60 mg/kg baicalin). For the FST, rats were also assigned to seven experimental groups (vehicle control, 10 mg/kg FLU or 6.25, 12.5, 25, 50, or 100 mg/kg baicalin). In the CMS model, rats were housed singly and maintained in the same environment as that used for the FST. For the CMS model, six experimental groups were included: vehicle control, stress, 20 mg/kg FLU, and 12.5, 25, or 50 mg/kg baicalin. Each experimental group consisted of ten animals. In the MAO assay, 30 rats were divided into six equal experiments: vehicle control, stress, 20 mg/kg FLU, and 12.5, 25, or 50 mg/kg baicalin. Each experimental group consisted of five animals. The animals were housed under controlled conditions of light (12 h light/dark cycle, lights on at 7:00 a.m.) and temperature (25 ± 1°C) with free access to food and water. All the animals were purchased from the Experimental Animal Centre of China Pharmaceutical University. Experiments followed a protocol approved by the local animal ethics committee and the local government. All experiments were in accordance with the recommendations of the European Union regarding animal experimentation (Directive of the European Council 86/609/EC).
Tail suspension test
Mice were given baicalin (10, 20, 40, 80, or 160 mg/kg p.o.) or vehicle (distilled water) once daily for 7 days. FLU (20 mg/kg p.o.) was also administered orally for successive 7 days as a positive control. The test was performed 1 h after the last drug administration. TST was similar to that described by Steru et al. (Citation1985). The mice were suspended by the tail to the edge of a shelf 75 cm above the floor. The tail was secured to the shelf by adhesive tape placed approximately 1 cm from the tip of the tail. The duration of immobility was recorded only during the last 4 min of the total 6-min test period. Mice were considered immobile only when they hung passively and completely motionless.
Forced swimming test
Mice were placed in individual glass cylinders (20 cm height × 10 cm diameter) containing 10 cm of water at 22–25°C for 6 min. The duration of immobility was scored during the last 4 min of the 6-min test period. A mouse was recorded as immobile when floating motionless or making only those movements necessary to keep its head above water. Mice were given baicalin (10, 20, 40, 80, or 160 mg/kg p.o.) distilled water, or FLU (20 mg/kg p.o.) once daily for 7 days. The last dose was given 1 h before the test. In this procedure for rat experiments, the animals were placed in clear glass cylinders (40 cm height × 18 cm diameter) filled with water (22–25°C) to a depth of 23 cm for 15 min. The rats were then dried and returned to their home cages. Before the test session, the rats were given baicalin (6.25, 12.5, 25, 50, or 100 mg/kg p.o.), distilled water, or FLU (20 mg/kg) for 7 successive days. In the test session, the rats were exposed to the cylinders as described above. The duration of immobility was scored during the last 4 min of the 6-min test period.
Measuring locomotor activity
In order to detect any association of immobility in the immobility test with changes in motor activity, the activities of animals treated with baicalin were tested in an open field test. Mice were randomly assigned to five equal groups (vehicle control, 20 mg/kg FLU, or 20, 40, or 80 mg/kg baicalin), and each experimental group consisted of ten mice. Each mouse was placed in the center of the open field apparatus, and the locomotor activity was assessed immediately before the FST. The open field apparatus was a field, 70 cm in diameter, which was demarcated into 18 approximately equal areas. Hand-operated counters were used to score locomotion (number of line crossings within 2 min) and rearing frequencies (number of times an animal stood on its hind legs). A separate researcher, who was blind to the treatment group, scored the behavior in the open field. The open field apparatus was washed with a deodorant solution (0.5% ammonia, 15% ethanol, 10% extran, 5% isopropanol, 10% pinol, and 59.5% water) before each behavioral test to eliminate possible odor clues left by previous subjects. Experiments were performed in a dark room, and the apparatus was illuminated by a 60-W bulb positioned 1 m above the center of the circle (Kim et al., Citation2005).
Chronic mild stress model and sucrose consumption test
Baicalin, at the doses of 12.5, 25, and 50 mg/kg was effective in the FST, and was further evaluated using the CMS model in rats. The animals were first trained to consume a 1% sucrose solution; training consisted of seven 1-h baseline tests (twice weekly) in which sucrose was presented, in the home cage, following a 14-h period of food and water deprivation; the sucrose intake was measured by weighing preweighed bottles containing the sucrose solution at the end of the test. Subsequently, sucrose consumption was monitored, under similar conditions, at weekly intervals throughout the entire experiment. On the basis of their sucrose intakes in the final baseline test, the rats were divided into two matched groups. One group of animals was subjected to the chronic mild stress procedure for a period of 8 consecutive weeks. Each week of stress regime consisted of two periods of food or water deprivation, two periods of 45°C cage tilt, two periods of intermittent illumination (lights on and off every 2 h), two periods of soiled cage (250 ml water in sawdust bedding), two periods of paired housing, two periods of low-intensity stroboscopic illumination (150 flashes per min), and two periods of no stress. All stressors were 10–14 h of duration and were applied individually and continuously day and night. Control animals were housed in separate rooms and had no contact with the stressed animals. They were deprived of food and water for the 14 h preceding each sucrose test, but otherwise food and water were freely available in the home cage.
On the basis of their sucrose intake after 3 weeks of stress, both stressed and control animals were divided further into matched subgroups (n = 6) and, for the subsequent 5 weeks, they received daily oral administrations of vehicle (1 ml/kg), FLU (20 mg/kg), or 12.5, 25, or 50 mg/kg baicalin. All drug administrations were given at 10:00 a.m. and the sucrose tests were carried out 24 h after the last drug treatment.
MAO assay
The MAO assay was started in rats after 3 weeks of stress in the CMS model. Rats were divided into one control and five stressed groups: vehicle, FLU (20 mg/kg), and 12.5, 25, or 50 mg/kg baicalin. The group of stressed animals was subjected to the chronic mild stress procedure for a period of 3 consecutive weeks. Each group consisted of five rats. The stressors used for MAO assay were according to the regime in sucrose intake test. MAO activity was assessed spectrophotometrically as described previously (Charles et al., Citation1977). Rat brain mitochondrial fraction was prepared following the procedure described previously (Schurr & Livne, Citation1976). Briefly, the mitochondrial fraction suspended in 10 vol. of cold sodium phosphate buffer (10 mM, pH 7.4, containing 320 mM sucrose), was mingled at 4°C for 20 min. The mixture was centrifuged at 1500 × g. for 30 min at 0°C and the pellets were resuspended in the same buffer. The protein concentration was adjusted to 1 mg/ml. Protein concentration was estimated by the Lowry method (Lowry et al., Citation1951) using bovine serum albumin as the standard. The assay mixtures contained 4 mM 5-HT or 2 mM benzylamine as specific substrates for MAO A and B, respectively, 250 µl solution of the mitochondrial fraction, and 10 mM sodium phosphate buffer (pH 7.4) up to a final volume of 1 ml. The reaction was allowed to proceed at 37°C for 20 min and was stopped by adding 1 M HCl (200 µl); the reaction product was extracted with 5 ml of butylacetate (for MAO A assay) or cyclohexane (for MAO B assay), respectively. The organic phases were measured at wavelength 280 nm for MAO A assay and 242 nm for MAO B assay with spectrophotometer, respectively. Blank samples were prepared by adding 1 M HCl (200 µl) prior to reaction and worked up subsequently in the same manner. Enzyme activity was expressed as nmol/min per milligram of protein.
Statistical analysis
The raw data were analyzed statistically. All the results are expressed as the means ± SEM and significance calculated using one-way analysis of variance (ANOVA) following by Student-Newman-Keuls test for the comparisons of means. Statistical differences were considered significant at p < 0.05.
Results
Antidepressant-like effect of baicalin in mice
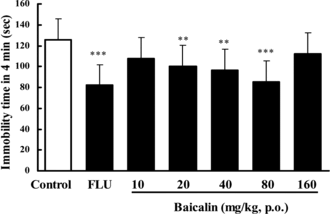
In the TST, pretreatment of the mice once daily for 7 days with baicalin at doses of 20, 40, or 80 mg/kg (p.o.) produced significant reductions in the duration of immobility, by 29.1%, 30.5%, or 31.5%, respectively (). Doses lower than 10 mg/kg or higher than 160 mg/kg are ineffective in this test. Treatment with baicalin at the dose of 20, 40, or 80 mg/kg (p.o.) for 7 days also reduced the immobility time by 20.1%, 23.1%, or 32.1% in the FST in mice (). The lower (10 mg/kg)and higher (160 mg/kg) doses were ineffective. FLU (20 mg/kg p.o.) reduced the immobility time by 34.7–42.3% in these experiments.
Figure 2 Effect of baicalin on immobility time in the tail suspension test in mice. In baicalin groups, mice were treated with baicalin (p.o.) once a day for 7 days. The test was performed 1 h after the last administration of baicalin. In the positive control, fluoxetine (FLU) was also given once daily for 7 days (20 mg/kg, p.o.). Data are expressed as means ± SEM. **p < 0.01, ***p < 0.001 vs. control.
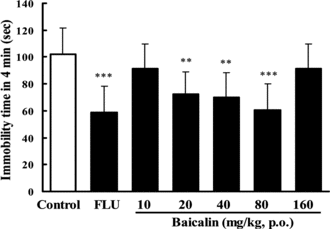
Figure 3 Effect of baicalin on immobility time in the forced swimming test in mice. In baicalin groups, mice were treated baicalin (p.o.) once a day for 7 days. The test was performed 1 h after the last administration of baicalin. In the positive control, fluoxetine (FLU) was also given once daily for 7 days (20 mg/kg, p.o.). Data are expressed as means ± SEM. **p < 0.01, ***p < 0.001 vs. control.
Antidepressant-like effect of baicalin in rats
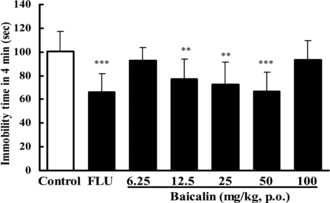
After 12.5, 25, or 50 mg/kg (p.o.; ) baicalin was administered, the immobility time in the FST in rats was also significantly reduced by 23.2%, 27.5%, or 33.9% (p.o.) in a dose-dependent manner. Doses lower than 6.25 mg/kg or higher than 100 mg/kg were ineffective in this test (p > 0.05). FLU (20 mg/kg p.o.) reduced the immobility time by 34.4% in this test.
Figure 4 Effect of baicalin on immobility time in the forced swimming test in rats. In baicalin groups, mice were treated with baicalin (p.o.) once a day for 7 days. The test was performed 1 h after the last administration of baicalin. In the positive control, fluoxetine (FLU) was also given once daily for 7 days (20 mg/kg, p.o.). Data are expressed as means ± S.E.M. **p < 0.01, ***p < 0.001 vs. control.
Effect of baicalin on the spontaneous motor activity in mice
In order to determine whether baicalin (at the dose of 20, 40, or 80 mg/kg) really has an antidepressant-like action, we needed to find whether baicalin had a significant effect on the central nervous system. The 20–80 mg/kg (p.o.) baicalin did not affect the spontaneous motor activity in mice as shown in , showing that baicalin had no effect on the central nervous system at least at the doses of 20–80 mg/kg (p.o.). All these results suggest that baicalin has an antidepressant-like effect in mice and rats.
Table 1.. Effects of 7-day treatments with baicalin and fluoxetine (FLU) on the open field parameters recorded for 2 min in mice.
Effect of baicalin on sucrose consumption in CMS model
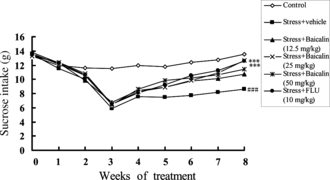
Chronic mild stress caused a gradual decrease in the consumption of 1% sucrose solution [F(5,54) = 16.98, p < 0.001] after 3 weeks of stress. In the stressed animals, treatment with baicalin (25 mg/kg) or FLU (20 mg/kg) caused a gradual recovery in sucrose intake compared with the sucrose intake at week 3. Stressed animals treated with FLU [F(4,54) = 13.27, p < 0.001], baicalin (12.5 mg/kg) [F(2,54) = 2.71, p > 0.05], baicalin (25 mg/kg) [F(5,54) = 11.95, p < 0.00)], and baicalin (50 mg/kg) [F(3,54) = 3.34, p > 0.05] for 5 weeks showed significant recovery compared with the vehicle-treated stressed animals ().
Figure 5 Sucrose consumption in stressed animals treated daily with baicalin and fluoxetine (FLU). The values are means (n = 10). Statistically significant differences indicated by ###p < 0.001 for stressed animals vs. control; **p < 0.01 and ***p < 0.001 for drug-treated stressed groups are relative to vehicle-treated stressed animals (VEH) at the indicated time points.
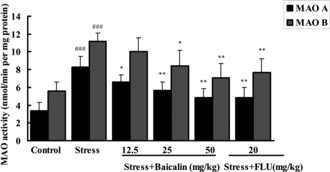
Effect of baicalin on the activities of MAO A and B in rat whole-arm fusion brain
The effects of baicalin and FLU for 21 days on the MAO A and B activities in rat whole brain are shown in . The MAO A and B activities in normal group were 3.39 ± 0.92 and 5.6 ± 0.99 nmol mg protein−1 min−1 respectively. After 21 days of stress, the activities of MAO A and B increased markedly and up to 8.31 ± 1.21 and 11.16 ± 0.94 nmol mg protein−1 min−1 (p < 0.001). Oral administration of baicalin at the doses of 12.5, 25, and 50 mg/kg significantly inhibited MAO A activity in a dose-dependent manner, producing 20.9%, 31.9%, and 41.4% inhibition. However, only baicalin at the doses of 25 and 50 mg/kg was significantly exhibited to inhibit MAO B activity, producing 24.7% or 36.5% inhibition. The effect of baicalin at the dose of 25 mg/kg was more potent than that of the positive control FLU (20 mg/kg) in MAO B inhibition. FLU at the dose of 20 mg/kg significantly reduced the MAO A and B activity by 41.9% and 31.3%, respectively, in this study.
Discussion
The tail suspension and forced swimming tests are two behavioral tests in rodents that predict the clinical efficacy of many types of antidepressant treatments (Porsolt et al., Citation1977Citation1978; Butterweck et al., Citation1998). In present study, we investigated the anti-immobility activity of baicalin is at 10, 20, 40, 80, or 160 mg/kg in mice and at 6.25, 12.5, 25, 50, or 100 mg/kg in rats in TST and FST. The results revealed that baicalin at oral doses 20, 40, or 80 mg/kg in the TST and the FST in mice and 12.5, 25, or 50 mg/kg in the FST in rats for 7 days significantly decreased the duration of immobility. In TST and FST, baicalin, at the lower dose of 10 mg/kg and higher dose of 160 mg/kg in mice or at the lower dose of 6.25 mg/kgand higher dose of 100 mg/kg in rats, was ineffective. This suggested determination of the effective dose scale of baicalin from 20 to 80 mg/kg in mice and 12.5 to 50 mg/kg in rats in the immobility test. As changes in immobility may be due to changes in locomotor activity caused by central nervous system stimulating agents, mice were tested in the open field test. Neither baicalin nor fluoxetine, at the doses tested, produced a significant effect on locomotor activity. These data show that baicalin has a specific antidepressant-like effect in animal models of immobility tests.
There are several procedures that employ stress in order to simulate depression in animals, of which the chronic mild stress (CMS) model has been most extensively validated and investigated. In the CMS model, rats or mice are exposed sequentially to a variety of mild stressors, which change every few hours over a period of weeks or months. This procedure causes, among other behavioral, biochemical, and physiological impairments, a substantial and long-lasting decrease in responsiveness to rewarding stimuli, which can be effectively reversed by chronic treatment with various antidepressant drugs and repeated application of electroconvulsive shocks. Conversely, non-antidepressant drugs, such as chlordiazepoxide, haloperidol, chlorprothixene, amphetamine, and morphine have been found to be ineffective in the CMS paradigm (Willner, Citation1997). A variety of neurochemical systems have been examined as a basis for the mechanism of the CMS-induced deficit in sensitivity to reward, and the most pronounced and consistent changes have been found within dopaminergic and serotonergic systems. For example, it was found that the CMS procedure causes an imipramine-reversible decrease in mesolimbic D2/D3 receptor binding and increase in cortical 5-HT2 receptor binding (Papp et al., Citation1994aCitationb). CMS also causes presynaptic changes in the function of both neurotransmitter systems, including increases in concentrations of DA and 5-HT and their metabolites, DOPAC and 5-HIAA, in the limbic region (Willner et al., Citation1991). Furthermore, in animals successfully treated with antidepressant drugs, behavioral recovery can be totally reversed by the acute administration of D2/D3 receptor antagonists (Willner, Citation1997), a further indication that these receptors are critically involved in stress-induced depression as exemplified in the CMS model. In the present study, CMS induced significant reductions in absolute and relative sucrose intake during weeks 0–3, whereas these effects were reversed by FLU and baicalin. Of note, the CMS is reported not to elicit consistent changes in the locomotor response in the open field (Harro et al., Citation1999). This also suggests that the recovery of sucrose consumption in the stressed animals by baicalin in our CMS study was not caused by a locomotor stimulant effect. It should be noted that the antidepressant-like effect of baicalin in the CMS model was not dose-dependent, only the intermediate dose of 25 mg/kgwas active. At the lower dose of 12.5 mg/kg and higher dose of 50 mg/kg, baicalin was ineffective against the CMS-induced deficit in sucrose consumption. There is no obvious explanation for this observation; it can only be speculated that the loss of activity at the higher dose (50 mg/kg) of baicalin in the CMS model could result from other than antidepressant-related effects as both the stressed and control animals receiving this dose showed signs of sedation, hypoactivity, and hyporeactivity. These effects were not severe as no significant decrease of the sucrose consumption was observed in control animals, and they disappeared after 3 weeks of treatment. Nevertheless, it cannot be excluded that these mild impairments, although no longer detected by gross behavioral observation, were sufficient to cause a lack of tolerance to the inhibition of antidepressant-like effect of higher doses of baicalin during its further treatment.
The antidepressant action of baicalin appears to result from the inhibition of MAO A and MAO B. MAO is an important enzyme in the metabolism of a wide range of monoamine neurotransmitters, including noradrenaline, dopamine, and 5-hydroxytryptamine. MAO exists in two forms, A and B. MAO A is more important than MAO B in the metabolism of the major neurotransmitter monoamines. MAO A inhibitors have been accepted to treat depression (Knoll, Citation1997; Wouters, Citation1998). In the present investigation, we have demonstrated that baicalin significantly inhibited in vivo. MAO A activity in rat whole brain in a dose-dependent manner, however, only baicalin at the dose of 25 or 50 mg/kg exhibited MAO B inhibitory activity. These findings suggested that antidepressant effect of baicalin in animal models of immobility tests may be related to the inhibitory activity of MAO, especially to that of MAO A.
It was reported that volumes of the double-side hippocampus were reduced in patients with major depression compared with healthy controls, and there was a positive correlation between hippocampus atrophy and the time course of the depression (Sapolsky, Citation2000aCitationb). Chronic psychosocial stress caused apical dendritic atrophy of hippocampal CA3 pyramidal neurons, which may be mediated by activation of the hypothalamic-pituitary-adrenal (HPA) axis acting in concert with the endogenous excitatory amino acid release (Magarinos et al., Citation1996; Sapolsky, Citation2000aCitationb). More and more studies have found that classical antidepressants protected the primarily cultured rat hippocampal neurons from the lesion induced by glucocorticoids, which was consistent with the results in PC12 cells (Li et al., Citation2003a). The antioxidant activities of baicalin have been confirmed (Hamada et al., Citation1993; Gao et al., Citation1996; Yoshino & Murakami, Citation1998). Gao et al. (Citation1999) showed that baicalin can scavenge hydroxyl radical, DPPH radical, and alkyl radical, which might be one of the important factors that contribute to their neuroprotective activities because they may neutralize free radicals generated by Glu/NMDA stimulation. Furthermore, Lee et al. (Citation2003) demonstrated that baicalin was effective on neuronal survival in primary cultured central neurons against glutamate-induced neuronal death. All of this evidence supports the hypothesis that the cytoprotective action is one of the common pathways of antidepressants (Kroczka et al., Citation2000; Petrie et al., Citation2000; Li et al., Citation2003aCitationb). It is therefore suggested that the antidepressant activity of baicalin may be mediated through the neuroprotective effect in central neurons.
In summary, the present study demonstrated that baicalin, a flavone extracted from Scutellaria baicalensis., has an antidepressant-like effect in behavioral models in mice and rats, and its antidepressant-like activity is in part mediated through the inhibition of MAO, particularly the activity of MAO A. Although the exact mechanism of baicalin on the antidepressant properties is not fully understood, baicalin could be promising as a potential candidate or an adjuvant to the conventional therapeutic modalities for depression.
Acknowledgment
This research was supported by the Jiangsu Natural Science Foundation (BK2005149), Jiangsu, People's Republic of China.
References
- Butterweck V, Petereit F, Winterhoff H, Nahrstedt, A (1998): Solubilized hypericin and pseudohypericin from Hypercum perforatum. exert antidepressant activity in the forced swimming test. Planta Med 64: 291–294.
- Charles M, McEwen J, Tabor CW, (Eds.) (1977): Methods in Enzymology, XVIIB.. New York and London, Academic Press, pp. 692–698.
- Chen ZY, Su YL, Lau CW, Law WI, Huang Y (1999): Endothelium-dependent contraction and direct relaxation induced by baicalin in rat mesenteric artery. Eur J Pharmacol 374: 41–47.
- Chen ZY, Su YL, Bi YR, Tsang SY, Huang Y (2000): Effect of baicalein and facetone extract of Scutellaria baicalensis. on canola oil oxidation. J Am Oil Chem Soc 77: 73–78.
- Fukutake M, Yokota S, Kawamura H, Iizuka A, Amagaya S, Fukuda K, Komatsu Y (1998): Inhibitory effect of Coptidis rhizoma. and Scutellariae radix. on azoxymethane-induced aberrant crypt foci formation in rat colon. Biol Pharm Bull 21: 814–817.
- Gabrielska J, Oszmianski J, Zylka R, Komorowska M (1997): Antioxidant activity of flavones from Scutellaria baicalensis. in lecithin liposomes. Z Naturforsch 52: 817–823.
- Gao D, Sakurai K, Chen J, Ogiso T (1995): Protection by baicalein against ascorbic acid-induced lipid peroxidation of rat liver microsomes. Res Commun Mol Patho Pharmacol 90: 103–114.
- Gao D, Sakurai K, Katoh M, Chen J, Ogiso T (1996): Inhibition of microsomal lipid peroxidation by baicalein: A possible formation of an iron-baicalein complex. Biochem Mol Biol Int 39: 215–225.
- Gao Z, Huang K, Yang X, Xu H (1999): Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi.. Biochim Biophys Acta 1472: 643–650.
- Hamada H, Hiramatsu M, Edamatsu R, Mori A (1993): Free radical scavenging action of baicalein. Arch Biochem Biophys 306: 261–266.
- Harro J, Haidkind R, Harro M, Modiri AR, Gillberg PG, Pahkla R, Matto V, Oreland L (1999): Chronic mild unpredictable stress after noradrenergic denervation: Attenuation of behavioral and biochemical effects of DSP-4 treatment. Eur Neuropsychopharm 10: 5–16.
- Huang HC, Wang HR, Hsieh LM (1994): Antiproliferative effect of baicalein, a flavonoid from a Chinese herb, on vascular smooth muscle cell. Eur J Pharmacol 25: 191–193.
- Huang KC (1999): Antibacterial, antiviral, and antifungal herbs. In: Huang KC, ed., The Pharmacology of Chinese Herbs, Boca Raton, FL, CRC Press, pp. 385–386.
- Kato M, Liu W, Yi H, Asai N, Hayakawa A, Kozaki K, Takahashi M, Nakashima I (1998): The herbal medicine Sho-saiko-to. inhibits growth and metastasis of malignant melanoma primarily developed in ret-transgenic mice. J Invest Dermatol 111: 640–644.
- Kim SH, Jin H, Seog DH (2005): Antidepressant effect of Chaihu-Shugan-San extract and its constituents in rat models of depression. Life Sci 76: 1297–1306.
- Kimura Y, Yokoi K, Matsushita N, Okuda H (1997): Effects of flavonoids isolated from Scutellariae radix. on the production of tissue-type plasminogen activator and plasminogen activator inhibitor-1 induced by thrombin and thrombin receptor agonist peptide in cultured human umbilical vein endothelial cells. J Pharm Pharmacol 49: 816–822.
- Kitamura K, Honda M, Yoshizaki H, Yamamoto S, Nakane H, Fukushima M, Ono K, Tokunaga T (1998): Baicalin, an inhibitor of HIV-1 production in vitro. Antiviral Res 37: 131–140.
- Knoll J (1997): History of deprenyl:/the first selective inhibitor of monoamine oxidase type B. Voprosy Meditsinskoi Khimii 43: 482–493.
- Kroczka B, Zieba A, Dudek D, Pilc A, Nowak G (2000): Zinc exhibits an antidepressant-like effect in the forced swimming test in mice. Polish J Pharmacol 52: 403–406.
- Kubo M, Matsuda H, Tani T, Arichi S, Kimura Y, Okuda H (1985): Studies on Scutellariae radix.. XII. Anti-thrombic actions of various flavonoids from Scutellariae radix.. Chem Pharm Bull 33: 2411–2415.
- Kyo R, Nakahata N, Sakakibara I, Kubo M, Ohizumi Y (1998): Baicalin and baicalein, constituents of an important medicinal plant, inhibit intracellular Ca2+ elevation by reducing phospholipase C activity in C6 rat glioma cells. J Pharm Pharmacol 50: 1179–1182.
- Lee H, Yang L, Wang C, Hu S, Chang S, Lee Y (2003): Differential effects of natural polyphenols on neuronal survival in primary cultured central neurons against glutamate- and glucose deprivation-induced neuronal death. Brain Res 986: 103–113.
- Li YF, Liu YQ, Huang WC, Luo ZP (2003a): Cytoprotective effect is one of common action pathways for antidepressants. Acta Pharmacol Sin 24: 996–1000.
- Li YF, Gong ZH, Cao JB, Wang HL, Luo ZP, Li J (2003b): Antidepressant-like effect of agmatine and its possible mechanism. Eur J Pharmacol 469: 81–88.
- Lin CC, Shieh DE (1996): The anti-inflammatory activity of Scutellaria rivularis. extracts and its active components, baicalin, baicalein and wogonin. Am J Chin Med 24: 31–36.
- Lin D, Bruijnzeel AW, Schmidt P, Markou A (2002): Exposure to chronic mild stress alters thresholds for lateral hypothalamic stimulation reward and subsequent responsiveness to amphetamine. Neuroscience 114: 925–933.
- Lowry OH, Rosebrough NJ, Far AL, Randall RJ (1951): Protein measurement with Folin phenol reagent. J Biol Chem 193: 265–275.
- Magarinos AM, McEwen BS, Flugge G, Fuchs E (1996): Chronic psychosocial stress caused apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci 16: 3534–3540.
- Matsuzaki Y, Kurokawa N, Terai S, Matsumura Y, Kobayashi N, Okita K (1996): Cell death induced by baicalein in human hepatocellular carcinoma cell lines. Jpn J Cancer Res 87: 170–177.
- Motoo Y, Sawabu N (1994): Antitumor effects of saikosaponins, baicalin and baicalein on human hepatoma cell lines. Cancer Lett 86: 91–95.
- Nagai T, Suzuki Y, Tomimori T, Yamada H (1995): Antiviral activity of plant flavonoid, 5,7,40-trihydroxy-8-methoxy flavone, from the roots of Scutellaria baicalensis. against influenza A (H3N2) and B viruses. Biol Pharm Bull 18: 295–299.
- Nakahata N, Kutsuwa M, Kyo R, Kubo M, Hayashi K, Ohizumi Y (1998): Analysis of inhibitory effects of Scutellariae radix. and baicalein on prostaglandin E2 production in rat C6 glioma cells. Am J Chin Med 26: 311–323.
- Okita K, Li Q, Murakamio T, Takahashi M (1993): Anti-growth effects with components of Sho-saiko-to (TJ-9) on cultured human hepatoma cells. Eur J Cancer Prev 2: 169–175.
- Papp M, Klimek V, Willner P (1994a): Parallel changes in dopamine D2 receptor binding in limbic forebrain associated with chronic mild stress-induced anhedonia and its reversal by imipramine. Psychopharmacology (Berl). 115: 441–446.
- Papp M, Klimek V, Willner P (1994b): Effects of imipramine on serotonergic and beta-adrenergic receptor binding in a realistic animal model of depression. Psychopharmacology 114: 309–314.
- Petrie RXA, Reid IC, Stewart CA (2000): The N.-methyl-D-aspartate receptor, synaptic plasticity, and depressive disorder: A critical review. Pharmacol Ther 87: 11–25.
- Porsolt RD, Le Pichon M, Jalfre M, Chatterjee SS (1977): Depression: A new animal model sensitive to antidepressant treatments. Nature 266: 730–732.
- Porsolt RD, Anton G, Blavet N, Jalfre M (1978): Behavioral despair in rats: A new model sensitive to antidepressant treatments. Eur J Pharmacol 47: 379–391.
- Qu R, Meng HB, Chu W (2003): Effect of Chaihu-Jia-Longgu-Muli decoction on monoamine neurotransmitters in the depression rat brain. Pharmacology and Clinics of Chinese Materia Medica 19: 1–3.
- Sapolsky RM (2000a): Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 57: 925–935.
- Sapolsky RM (2000b): The possibility of neurotoxicity in the hippocampus in major depression: A primer on neuron death. Biol Psychiat 15: 713–714.
- Schurr A, Livne A (1976): Different inhibition of mitochondrial monoamine oxidase from brain by hashish components. Biochem Pharmacol 25: 1201–1203.
- Shi HL, Zhao BL, Xin WJ (1995): Scavenging effects of baicalin on free radicals and its protection on erythrocyte membrane from free radical injury. Biochem Mol. Biol Int 35: 981–994.
- Steru L, Chermat R, Thierry B, Simon P (1985): The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berlin) 85: 367–370.
- Willner P, Klimek V, Golembiowska K, Muscat R (1991): Changes in mesolimbic dopamine may explain stress induced anhedonia. Psychobiology 19: 79–84.
- Willner P (1997): Validity, reliability and utility of the chronic mild stress model of depression: A 10 years review and evaluation. Psychopharmacology 134: 319–329.
- Wouters J (1998): Structural aspects of monoamine oxidase and its reversible inhibition. Curr Med Chem 5: 137–162.
- Yano H, Mizoguchi A, Fukuda K, Haramaki M, Ogasawara S, Momosaki S, Kojiro M (1994): The herbal medicine Sho-saiko-to. inhibits proliferation of cancer cell lines by inducing apoptosis and arrest at the G0/G1 phase. Cancer Res 54: 448–454.
- Yoshino M, Murakami K (1998): Interaction of iron with polyphenolic compounds: Application to antioxidant characterization. Anal Biochem 257: 40–44.