Abstract
The in vivo. evaluation of toxicological and antifungal activity of the Mentha. x piperita. L. essential oils and menthol was made on 2-month-old male Wistar rats. We examined the therapeutic potency against experimentally induced dermatomycoses in rats using the most frequent dermatomycetes, Trichophyton mentagrophytes., T. rubrum., and T. tonsurans.. The therapeutic efficacy of 1% solution of essential oil and menthol, as well as commercial preparation bifonazole, was evaluated. During the 36-day observation period, the oil-treated animals were cured completely.
Introduction
Human infections have markedly increasing during the past 10 years, especially in immunocompromised patients. Consequently, as high as 10% of hospital-acquired systemic infections are caused by fungi.
Among animal and human pathogens, dermatomycetes are the main cause of dermatomycoses (hair, skin, and nails infections). Superficial infections are not life threatening, but chronic fungal infections of the skin carry a considerable morbidity (Bell-Syer et al., Citation1998). The increasing resistance of human pathogens to current commercial drugs is a serious medical problem and has resulted in the need for novel antimicrobial agents.
Natural products derived from plants have traditionally been used in ethnomedicine. In Western medicine, substances derived from vascular, especially flowering plants, constitute about 25% of prescribed remedies, and 74% of the 121 bioactive plant-derived compounds currently in worldwide use were identified via research based on leads from etnomedicine (Sokmen et al., Citation1999). Recent research showed that higher plants may serve as promising sources of novel antimycotics with no side effects on human beings and animals (Clark & Hufford, Citation1993). Essential oils play a great role in these investigations. Studies over the past hundred years have demonstrated the antimicrobial properties of several common spice oils (Bullerman et al., Citation1977). Some authors (Maruzzela & Balter, Citation1959) found that 100 essential oils out of 119 spice oils tested possessed an antagonistic effect to at least 1 of 12 pathogenic fungi, and 50 of these samples showed a wide spectrum of activity against all fungi tested. Previous in vitro. and in vivo. investigation of antifungal activity of the essential oils of Origanum vulgare. subsp. hirtum., Mentha spicata., Lavandula angustifolia., and Salvia fruticosa. suggested that they could be used as antifungal agents (Adam et al., Citation1998).
The purpose of this study was to investigate Mentha. x piperita. essential oil for potential antifungal activity. The selection of plant for evaluation was based on traditional use of this plant in treatment of various infection diseases (Jančić et al., Citation1995; Kovačević, Citation2000).
Mentha. species have been credited with a long list of pharmacological properties, especially as antimicrobial agents (Hitokoto et al., Citation1980; Jansen et al., Citation1986; Crespo et al., Citation1990; Panizzi et al., Citation1993; Reddy et al., Citation1998). But, there is only limited information in the literature on the antifungal activity of essential oils toward human fungal pathogens in vivo..
In the current study, the antifungal activity of Mentha. x piperita. essential oil and menthol was examined against widespread pathogenic fungal strains Trychophyton. mentagrophytes., T. rubrum., and T. tonsurans., which cause superficial skin infections in human beings.
Materials and Methods
Plant material and isolation of essential oil
Mentha. x piperita. L. was identified at the Institute of Botany, Faculty of Biology, University of Belgrade. Material was collected at the experimental field of the Institute for Medicinal Plant Research “Josif Pančić.”
Composition of essential oils was investigated using analytical GC/FID and GC/MS techniques. For this purpose, a HP 5890 series II gas chromatograph, equipped with split-splitless injector, fused silica capillary column (25 m × 0.32 mm), coated with cross-linked methyl silicone gum (0.5-µm film thickness), and FID was employed. Essential oil solutions in ethanol (1%) were injected in split mode (1:30). The injector was heated at 250°C, FID at 300°C, while column temperature was linearly programmed from 40°C to 280°C (4°C/min).
GC/MS analysis was carried out on a HP-GCD, equipped with a split-splitless injector, fused silica capillary column (50 m × 0.2 mm) PONA, coated with cross-linked methyl silicone gum (0.5-µm film tickness) and mass selective detector. The chromatographic conditions were as above. Transfer line (MSD) was heated at 280°C. EMS spectra (70 eV) were acquired in scan mode in m./e. range 40–300.
Identification of individual constituents was carried out by comparison of their retention times with those of analytical standards and by computer searching and matching the mass spectral data with those held in the Wiley/NBS library of mass spectra. For quantification purposes, area percent reports obtained by FID were used.
An essential oil constituent, menthol, was a commercial standard (Aldrich Chemical Co., Milwaukee, WI, USA).
Animals
Two-month-old male Wistar rats were acclimated for 1 week prior to treatment at 21°C, 50% humidity, and 12-h light/dark cycle at the institute's facility. Rats were allowed access to food and water ad libitum.. Protocols for animal use followed the Public Health Service Policy on Human Care and Use of Laboratory Animals and were approved by the institutional animal care and use committee.
Toxicology
In order to determine the nontoxic concentration of the essential oil and component investigated, we used five male Wistar rats (17–24 g). A stock solution (0.5 ml) of essential oil and component diluted in 0.9% NaCl solution (0.01–1% v/v) was injected intraperitoneally in five male Wistar rats (Pharmacopoeia Jugoslavica, Citation1984). The concentrations that were nontoxic for the animals investigated were used for further research.
In vivo. fungitoxicity assay
The in vivo. investigation of antifungal activity of Mentha. x piperita. essential oil, menthol, was made as described previously (Adam et al., Citation1998). Three dermatomycetes were used: Trychophyton mentagrophytes., T. rubrum., and T. tonsurans.. The organisms were isolated from patients of the Center for Preventive Medicine, MMA (Belgrade, Serbia and Montenegro).
The micromycetes were maintained on Sabouraud Dextrose Agar (SDA) containing 40 g glucose, 10 g agar, and 10 g peptone in 1 L of distillate H2O. The cultures were stored at 4°C and subcultured once a month (Booth, Citation1971).
On the back of each animal, areas of 4 cm2 were cleaned and depilated. The infectious inoculum was prepared from a 7-day-old culture of Trychophyton mentagrophytes., T. rubrum., and T. tonsurans.. The inoculum was applied on the backs of animals immediately after depilation and left for 3 days. The establishment of active infection was confirmed on the fourth day by isolation of the pathogens from skin scales cultured from infected loci on SDA plates containing 100 units/ml penicillin and streptomycin. Infections were, also, confirmed by visual examination of animals at the eighth to tenth days. In the animals in which active infections were confirmed, treatment was initiated on the 14th day postinoculation and continued until complete recovery from infection was achieved. The ointments contained 1% (v/v) of essential oil and component mixed in petroleum jelly. The commercial fungicide, bifonazole, was used as a control. The treatments were applied once a day, and the infected areas were scored visually for inflammation and scaling as well as for the presence of the pathogens by cultivating skin scales from infected loci in SDA plates containing 100 units/ml penicillin and streptomycin every day.
Results and Discussion
The qualitative and quantitative composition of the essential oil of Mentha. x piperita. L. is presented in . The essential oil obtained from this species is characterized by high content of menthol (37.40%) and menthon (12.70%).
Table 1.. Composition of essential oil of Mentha. x piperita..
The essential oil of peppermint as well as menthol was tested for their potential toxicological activity in 0.1% and 1% (v/v) solutions in ethanol and petroleum jelly, separately. There is no toxicological activity for 0.1% solutions on the rats. However, in this work, the animals were treated topically, and according to the literature (Adam et al., Citation1998), for further investigation 1% solutions were used.
The therapeutic efficacy of the ointments was evaluated daily by macroscopic examination of lesions and by screening for the presence of the infections by culturing skin scales from the infected area. The lesions were scored as cured only when the infected area was free of macroscopic lesions and the cultures were negative.
First symptoms (small vesicles) at the rats inoculated with T. mentagrophytes. were observed at the 8th day of the experiment, while later (20th day), these exhibited as bloody wounds, 20 mm in diameter ( and ). We started with the treatment at the 20th day of the experiment. At the 15th day of the treatment with solution of M.. x piperita. essential oil, the rats were completely cured, there were no visually observed symptoms, and cultures were negative (c). Animals treated with the commercial drug, bifonazole, were cured after 14 days of treatment.
Figure 1 Experimentally induced dermatomycoses in rats by T. mentagrophytes.. (a) Small vesicles at the rats observed at the eighth day of the experiment. (b) Bloody wounds at the 20th day of experiment. (c) Rat completely cured with M. piperita. essential oil after 15 days of treatment.

On the back of the rats infected with T. rubrum., first symptoms (scaly, erythematous to tawny-brown, bilateral and asymmetric lesions) were observed at the eighth day after the inoculation (a). We started with the treatment at the 20th day. On the animals treated with M.. x piperita. essential oil, after 10 days of treatment, the symptoms exhibiting a sharply marginated border studded with small vesicles. During the 30 days of treatment, rats were cured (b). The animals treated with bifonazole were cured during the 15-day treatment.
Figure 2 Experimentally induced dermatomycoses in rats by T. rubrum.. (a) Scaly, erythematous to tawny-brown, bilateral and asymmetric lesions observed at the eighth day after the inoculation. (b) There are no symptoms after 30 days of treatment with M. piperita. essential oil.

First symptoms (small, tawny-brown lesions) in the rats infected with T. tonsurans. were observed at the sixth day after the inoculation. Small lesions were formed behind the ears, too. After 18 days of treatment, the symptoms became worse and expanded to the ears ( and ). Treatment was started at the 20th day after the inoculation. Animals treated with the oil of M.. x piperita. were cured after 29 days of treatment (c). During that period, symptoms sometimes disappeared and appeared again, in several animals, but the cultures were positive, so we prolonged the treatment until the cultures were negative. Animals treated with bifonazole were cured after 14 days of treatment. All the animals treated with menthol were cured within 10 days.
Figure 3 Experimentally induced dermatomycoses in rats by T. tonsurans.. (a) Tawny-brown lesions and wounds at the ears observed at the 18th day after the inoculation. (b) Detail of infected ear. (c) Animals treated with the oil of M. piperita. were cured after 29 days of treatment.
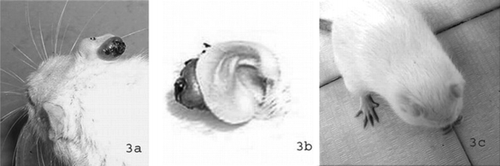
During the 30-day observation period, the treated animals were cured completely. In the rats infected with T. rubrum. and T. tonsurans. during the observation period, symptoms sometimes disappeared and appeared again, but the fungus was still recovered from the skin. It should be noted that in many cases, macroscopic lesions disappeared long before the elimination of the infectious agent, indicating that long treatment periods of application and evaluation are necessary (Adam et al., Citation1998). It is normal because dermatomycetes infections typically resolve on their own over a variable time period (18 months to 4 years) depending on the immune response. Many dermatomycetes rarely cause strong inflammatory reactions, making it very difficult for the immune system to recognize and eliminate the fungus.
It can be seen that clinical parameters were the most characteristic (bloody wounds) in the animals infected with T. mentagrophytes., but the period of treatment was shorter, while, in the rats infected with T. rubrum. and T. tonsurans., symptoms were much more temperate, but the duration of treatment was longer than in the previous case. Also, symptoms caused by T. rubrum. and T. tonsurans. disappeared several times during the experiment, and came back after a few days. The reason for such a phenomenon may be due to the zoophilic characteristic of T. mentagrophytes. and the antrophilic characteristic of T. rubrum. and T. tonsurans..
The essential oil of M.. x piperita. completely cured the animals infected with T. mentagrophytes. within 15 days, T. rubrum. within 30 days, and T. tonsurans. within 29 days.
The animals treated with the commercial drug, bifonazole, were cured after 14–15 days of treatment. If we compare the activity of this commercial drug and the essential oil investigated, the first possessed higher therapeutic activity, but as we commented earlier, commercial drugs could possess some side effects, while there were no side effects in animals treated with these secondary metabolites derived from plants. Also, pathogens could become resistant to this commercial drug if it is applied over a long period of time (Mitrović & Arsić-Arsenijević, Citation2000).
It can be seen that menthol possessed higher therapeutic and antifungal activities than the essential oil investigated. Also, menthol showed better activity than bifonazole.
Because oil showed lower activity than menthol, it is evident that properties of the active principles could be ascribed to this component. It appears that interactions of a chemical nature between oil constituents blocked the actions of the individual active principles as an antagonistic effect (Davidson & Parish, Citation1989).
From the above results it can be concluded that essential oil of Mentha. x piperita. and menthol showed very good therapeutic and antifungal effects in vivo.. These compounds could represent possible alternatives for the treatment of patients infected by dermatomycetes. Even more, because of the side effects of commercial fungicides and possible resistance of pathogens to the synthetic mycotics, the preparation with natural products has an advantage in treatment of fungal-caused diseases.
Acknowledgment
The authors are grateful to the Ministry of Science and Ecology for financial support (grants 1511 and 1544).
References
- Adam K, Sivropoulu A, Kokkini S, Lanaras T, Arsenakis M (1998): Antifungal activities of Origanum vulgare. subsp. hirtum, Mentha spicata, Lavandula angustifolia. and Salvia fruticosa. essential oils against human pathogenic fungi. J Agri Food Chem 46: 1739–1745.
- Bell-Syer SEM, Hart R, Crawford F, Tyrrel W, Torgerson D (1998): Systematic Treatments for Fungal Infections of the Skin of the Foot. Oxford, Cochrane Review Protocol, The Cochrane Library 3.
- Booth C (1971): Fungal culture media. In: Norris JR, Ribbons DW, eds., Methods in Microbiology IV. London & New York, Academic Press, pp. 49–94.
- Bullerman LB, Lieu Y, Seier SA (1977): Inhibition of growth and aflatoxin production by cinnarnon and clove oils, cinamic aldehyde and eugenol. J Food Sci 42: 1107–1109.
- Clark AM, Hufford CD (1993): Discovery and development of novel prototype antibiotics for opportunistic infections related to acquired immunodeficiency syndrome. In: Kinghorn AD, Balandrin MF, eds., Human Medicinal Agents from Plants. ACS Symposium Series 534. Washington, DC, American Chemical Society.
- Crespo EM, Jimenz J, Gomis E, Navaro C (1990): Antibacterial activity of the essential oil of Thymus serpylloides. subspecies gadorensis.. Microbies 61: 181–184.
- Davidson PM, Parish EM (1989): Methods for testing the efficacy of food antimicrobials. Food Technol 43: 148–155.
- Hitokoto H, Morozumi S, Wauke T, Sakal S, Kubata H (1980): Inhibitory effects of spices on growth and toxin production of toxigenic fungi. Appl Environ Microbiol 39: 818–822.
- Jančić R, Stošić D, Mimica-Đukić N, Lakušić B (1995): Aromatične biljke Srbije, Dečje novine, Beograd-Gornji Milanovac.
- Janssen MA, Chis JLN, Scheffer CJJ, Baerhein Svendsen A (1986): Screening for antimicrobial activity of some essential oils by the agar overlay technique. Pharmaceutisch Weekblad Scientific Edition 8: 289–292.
- Kovačević N (2000): Osnovi farmakologije, MUM, Beograd.
- Maruzzela JC, Balter J (1959): The action of essential oils on phytopathogenic fungi. Plant Dis Rep 43: 1143–1147.
- Mitrović S, Arsić-Arsenijević V (2000): Antifungal resistance in human mycoses. Introductory lecture, VIII Congress of Yugoslav Microbiologists, Vrnjačka banja, pp. 263–266.
- Panizzi L, Flamini G, Cioni LP, Moreli I (1993): Composition and antimicrobial properties of essential oils of four mediterranean lamiaceae. J Ethnopharmacol 39: 163–170.
- Pharmacopea Jugoslavica (1984): Investigation of non-toxic compounds. Belgride, Federal Office for Health Protection 4(1): 152.
- Reddy BYM, Angers P, Gosselin A, Arul J (1998): Characterization and use of essential oil from Thymus vulgaris. against Botrytis cinerea. and Rhizopus stolonifer. in strawberry fruits. Phytochemistry 47: 535–550.
- Sokmen A, Jones MB, Erturk M (1999): The in vivo antibacterial activity of Turkish medicinal plants. J Ethnopharmacol 67: 79–86.
