Abstract
Bioassay-guided isolation of the marine sponge Hippospongia sp. collected at Palau led to the isolation of three sesquiterpene quinone metabolites. They were determined as ilimaquinone (1), 5-epi.-ilimaquinone (2), and 5-epi.-isospongiaquinone (3) by NMR data analysis. Compounds 2 and 3 were obtained as a mixture (ca. 4:1). The cytotoxicity on human tumor cell lines of NCI-H460, HepG2, SF-268, MCF-7, HeLa, and HL-60, inhibitory effects on starfish oocytes maturation, and cell cycle arrest on HepG2 cell line for all compounds were evaluated.
Introduction
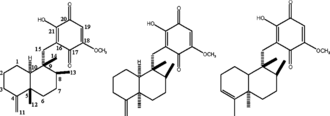
The ocean has been regarded as source of a large group of structurally unique natural products that are mainly accumulated in invertebrates such as sponges, tunicates, bryozoans, and mollusks. Several of these compounds showing pronounced pharmacological activities are interesting candidates for new drug developments (Munro et al., 1999; Marinlit, Citation2001; Blunt et al., Citation2004Citation2005). Cell cycle inhibitors have attracted much interest due to their potential as pharmaceutical agents. To discover novel cell cycle inhibitory agents, we applied a bioassay method using starfish oocytes as a model and occurrence of germinal vesicle breakdown (GVBD) as an indicator to screen the extracts of marine sponges collected at Palau. Inhibitors of starfish oocytes reinitiation and maturation were sought to obtain biologically active substances that might act on either hormonal signal transduction or cell cycle regulation, including cyclinB/cdc2 inhibitors and microtubule assembly regulators (Toraya et al., 1995). Marine Sponge Hippospongia sp. extract sample with strong inhibitory activity on starfish oocytes maturation was subjected to bioassay-guided fractionation. Three sesquiterpene quinine metabolites (1–3) () were isolated from this sponge. In this report, we describe the isolation, structure determination, cytotoxicity, and cell cycle inhibitory activities of these compounds.
Materials and Methods
General experimental procedures
1H NMR and 13C NMR spectra were recorded on a Bruker AVANCE spectrometer at 400 and 100 MHz with TMS as an internal standard. Optical rotations were obtained on a JASCO P1020 polarimeter (JASCO International Co. Ltd., Tokyo, Japan). Open column chromatography was conducted on silica gel (Kanto Chemical Co., Inc, Tokyo, Japan). The preparative HPLC was performed on a Shimadzu LC-10A apparatus equipped with a Shimadzu SPD-10A UV-Vis detector using an ODS column (Docsil, Senshu-pack, 6 × 250 mm, 5 µm). 3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), RNase A, and propidium iodide (PI) were purchased from Sigma (St. Louis, MO, USA). RPMI-1640 medium, fetal bovine serum (FBS), and trypsin-EDTA solution (1X) were obtained from GIBCO-BRL (Grand Island, NY, USA).
Material
Sponge Hippospongia sp. was collected by scuba diving in July, 2000, at Palau and kept in a freezer at −30°C until extraction. The voucher specimen (00-02-05 = 1–5) is deposited and identified by Professor Michio Namikoshi at the Department of Ocean Sciences, Tokyo University of Marine Science and Technology.
Extraction and isolation
The sponge (10 g) was thawed, cut into small pieces, and extracted with ethanol (2 × 100 ml). The ethanol extract was evaporated, and the residue was partitioned between water and EtOAc. The organic extract (0.2 g) was chromatographed on SiO2 (ϕ 2.5 × 15 cm) with hexane-ethyl actetate (gradient elution, 9:1, 8:2, 7:3, and 1:1) to give 12 fractions. The bioactivity was detected in fraction 5 eluted by hexane-ethyl actetate (8:2, 40 mg). The bioactive fraction was further separated by an ODS column with 80% MeOH/H2O (0.1% TFA) to afford ilimaquinone (1; 12 mg) and a mixture of 5-epi.-ilimaquinone and 5-epi.-isospongiaquinone (2 and 3; 10 mg).
Ilimaquinone (1), pale yellow powder; [α]25D -24° (c. 1.0, CHCl3); 1H NMR (CDCl3): δ.1.45 (1H, m, H-1), 2.08 (1H, d,10.8 Hz, H-1), 1.16 (1H, m, H-2), 1.86 (1H, bd, 9.6 Hz, H-2), 2.05 (1H, d, 10.8 Hz, H-3), 2.33 (1H, td,8.8, 4.4 Hz, H-3), 1.36 (1H, m, H-6), 1.52 (1H, d, 9.6 Hz, H-6), 1.38 (2H, m, H-7), 1.16 (1H, m, H-8), 0.77 (1H, d, 11.2 Hz, H-10), 4.44 (2H, d, 5.2 Hz, H-11), 1.04 (3H, s, H-12), 0.97 (3H, d, 8.0 Hz, H-13), 0.85 (3H, s, H-14), 2.50 (2H, q, 13.6 Hz, H-15), 5.85 (1H, s, H-19), 3.86 (3H, s, 18-OCH3), 7.49 (1H, s, 21-OH); 13C NMR (C-1-C-21): δ.23.2, 28.6, 33.0, 160.5, 40.5, 36.7, 27.9, 38.1, 43.3, 50.2, 102.5, 20.6, 17.8, 17.3, 32.4, 117.4, 182.0, 161.7, 102.0, 182.3, 153.3, 56.8 (18-OCH3). Negative ESI-MS: m/z. 357 [M-H]−.
5-epi.-Ilimaquinone (2) and 5-epi.-isospongiaquinone (3), yellow powder; 13C NMR (C-1-C-21, 2): δ. 22.5, 24.9, 32.0, 153.5, 39.5, 37.9, 27.8, 39.6, 44.9, 48.5, 105.8, 33.2, 18.3, 18.6, 32.7, 117.7, 182.2, 161.7, 102.0, 182.5, 153.5, 56.9; 13C NMR (C-1-C-21, 3): δ.18.2, 24.5, 123.9, 138.9, 44.6, 37.3, 29.1, 37.3, 38.9, 46.1, 19.7, 32.3, 19.1, 16.3, 32.8, 117.8, 182.2, 161.7, 102.0, 182.5, 153.4, 56.9. Negative ESI-MS: m/z. 357 [M-H].
Bioassay
Starfish oocyte assay (Liu et al., Citation2005)
The starfish Asterina pectinifera. was collected from the coastal waters off Japan during the breeding season and kept in artificial seawater (ASW; 10 mM EPPS-NaOH, pH 8.2, containing 0.46 M NaCl, 10 mM KCl, 35.9 mM MgCl2, 1.75 mM MgSO4, and 9.18 mM CaCl2). The starfish ovaries were excised and cut into pieces in calcium-free ASW, and then the attached follicle cells were removed by pipetting. Oocytes in ASW thus prepared were collected by low-speed centrifugation (850 × g., 2 s) and suspended in chilled ASW until use. Immature oocytes were counted and adjusted to 100 cells/ml. Oocytes were incubated on ice for 10 min with each test sample followed by the addition of 1-MA. After incubation at 20°C for 60 min, the state of germinal vesicles was observed under an inverted microscope. DMSO at a concentration of less than 1% was used to dissolve samples. This concentration of DMSO had no inhibitory effect on GVBD. d-α-Tocopheryl acid succinate (vitamin E) showed 100% inhibition of GVBD at 1 mM and was used as a positive control.
Cell culture
Human cancer cell lines NCI-H460 (lung), HepG2 (liver), SF-268 (CNS), MCF-7 (breast), HeLa (cervical), HL-60 (leukemia), and Hek-293 (human embryonic kidney) were obtained from the American Type Culture Collection (ATCC) and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 units/ml of penicillin, and 100 µg/ml of streptomycin. Cells were maintained at 37°C in a humidified atmosphere of 5% CO2 in air.
Growth inhibitory evaluation
The effect on cell viability was determined by the MTT method. Briefly, cells (1 × 105 cells/ml) were seeded in 96-well plates. After 24 h incubation to allow for attachment, the cells were incubated with or without various concentrations of test samples. Then, 10 µl of MTT (5 mg/ml) was added to each well and the plates were incubated for an additional 4 h at 37°C. The formazan crystals formed by MTT metabolism were solubilized by the addition of 150 µl of DMSO to each well, and the absorbance at 570 nm was measured with a microplate reader (Bio-Rad, CA, USA). The absorbance of control cells was considered as 100%. The result was expressed as a percentage, relative to control incubations, and the IC50 values were calculated using nonlinear regression analysis (percent survival vs. concentration). IC50 values are defined as the drug concentrations to inhibit 50% cell growth.
Cell cycle analysis (Wang et al., Citation2004)
The HepG2 cells treated with samples were harvested for different time periods, washed twice with ice-cold PBS, and then fixed overnight in 70% ice-cold ethanol. After digestion of RNA with 25 µg/ml RNase A for 30 min at 37°C, cells were collected by centrifugation and stained with PI water solution (50 µg/ml PI, 0.1% sodium citrate, and 0.2% Nonidet P-40 in water) for 30 min at 4°C under a lightproof condition. The cells were collected and analyzed with a FACScalibur flow cytometer (Becton Dickinson Co. Ltd., NJ, USA). Data collection and analysis of the cell cycle distribution were performed using CellQuest and the Modfit software (Becton Dickinson Co. Ltd., NJ, USA).
Results and Discussion
Sponges of the families Dysideidae, Thorectidae, Spongiidae, and Haploscleridae have been discovered to produce a variety of sesquiterpene, hydroquinone, and quinone metabolites (Capon, Citation1995). All of these compounds possess a 4,9-friedodrimane or a drimane skeleton, which is substituted with a hydroquinone or quinone moiety. They represent a group of still expanding C15-C6 metabolites with notable medicinal applications such as antitumor, antibacterial, and anti-HIV activities (Luibrand et al., Citation1979; Nakamura et al., Citation1986; Capon et al., Citation1987; Kondracki et al., Citation1989; Kushlan et al., Citation1989; Hirsch et al., Citation1991; McConnell et al., Citation1992; Rodriguez et al., Citation1992; Urban et al., Citation1992; Evans et al., Citation1994; Talpir et al., Citation1994; Shen et al., Citation1997; Goclik et al., Citation2000; Kwak et al., Citation2000; Salmoun et al., Citation2000; Mitome et al., Citation2001Citation2002; Utkina et al., Citation2003). Of particular interest are two antimitotic agents called avarol and avar-one, which were shown to inhibit HTLV-III replication in human H9 cells as measured by determination of reverse transcriptase. This result suggested that sesquiterpene quinones and hydroquinones may be useful in the treatment of AIDS. Due to their potential antitumor and anti-HIV activities as well as the novelty of their structures, the exploration of natural sesquiterpene quinones has continued to grow in the past decade and resulted in the discovery of more than 100 natural products of this type.
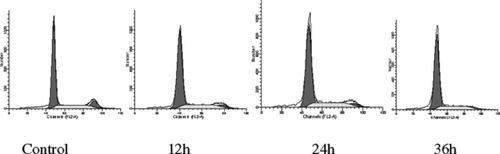
In the current study, we isolated three known sesquiterpene quinines, ilimaquinone (1), 5-epi.-ilimaquinone (2), and 5-epi.-isospongiaquinone (3), from a sponge collected in Palau. Compounds 2 and 3 were isolated as a mixture of an approximate ratio of 4:1, which was estimated from HPLC and NMR analyses. The structures were determined by NMR data analysis and comparison with literature values (Kondracki et al., Citation1989; Rodriguez et al., Citation1992; Salmoun et al., Citation2000). Both compound 1 and the mixture of compounds 2/3 showed strong activities in inhibiting the maturation of starfish oocytes, the proliferation of cancer cell lines, and arresting G0/G1 phase transition of the HepG2 cell line. Compounds 1 and 2/3 showed strong inhibitory activity on meiotic maturation of starfish oocytes with IC50 values of 5.6 and 7.0 µM, respectively. The integrate germinal vesicles (nucleolus) of oocytes were observed under a microscope when compared with negative control as shown in . The IC50 values were defined as the concentration at which 50% inhibition of GVBD was achieved. The growth inhibitory activity of compounds 1 and 2/3 on several human cancer cell lines, including NCI-H460 (lung), HepG2 (liver), SF-268 (CNS), MCF-7 (breast), HeLa (cervical), and HL-60 (leukemia), was examined by MTT methods (). Compound 1 presented strong inhibition on the proliferation of NCI-H460, HepG2, SF-268, 293, and HeLa cell lines with IC50 values of 7.1, 2.5, 6.3, 6.0, and 2.4 µg/ml, respectively. However, 1 was not active with HL-60. For MCF-7, there was no dose-dependent effect observed. The mixture of 2/3 showed the same growth inhibition spectrum as that of 1. Both 1 and 2/3 showed stronger activity to HepG2 and HeLa than others cell lines. They also presented strong cytotoxicity to Hek-293 (human embryonic kidney cell) taken as normal cell control, which indicates nonspecific cytotoxicity. The time-dependent cell cycle distribution profiles of HepG2 cells when treated with 10 µg/ml of ilimaquinone (1) were analyzed by flow cytometry. The cell cycle was arrested at the G0/G1 stage compared with the control, and the inhibition rate was enhanced when treatment time increased (; ) ). The dose-dependent cell cycle distribution profile of HepG2 cells treated with ilimaquinone (1) was analyzed as well. However, the cell cycle distribution percentages remained almost unchanged when the concentration of 1 was increased from 10 to 20 µg/ml. By microscopy observation, many crystals of 1 were found, which may contribute to poor dose-dependent effects.
Table 1.. Inhibitory activity of compounds 1 and 2/3 on proliferation of cancer cell lines.
Table 2.. Effects of ilimaquinone (1) (10 µg/ml) on cell-cycle distribution in HepG2 cells.
Figure 2 Inhibitory activity of ilimaquinone (1) on oocytes maturation of starfish A: negative control; B: positive control (Vitamin E, 1 mM); C: ilimaquinone (14 µM); and D: ilimaquinone (5.6 µM).
Figure 3 The time-dependent cell cycle distribution profiles of HepG2 cells treated with 10 µg/ml of ilimaquinone (1).
Ilimaquinone (1) was first isolated from the sponge Hippiospongia metachromia. in 1979 (Luibrand et al., Citation1979). After that, it has been found in many different species of sponges. A recent biological study revealed many important bioactivities of 1; i.e., induction of erythroid differentiation in K562 cells (Aoki et al., Citation2004), inhibition of the RNase H activity of HIV-1 reverse transcriptase (Loya et al., Citation1990Citation1993), antiproliferative effect on Leishmania mexicana. (Rangel et al., Citation1997), disassembly of dynamically unstable microtubules in fibroblasts and various epithelia cell lines (Pous et al., Citation1998), induction of complete vesiculation of Golgi apparatus (Takizawa et al., Citation1993), and inhibition of the cytotoxicities of ricin, diphtheria toxin, and other protein toxins in Vero cells (Nambiar et al., Citation1995). Our research provides further evidence for its anticancer activity, and it is the first published report on the cell cycle arrest activity on tumor cell line HepG2 and inhibitory activity on starfish oocytes maturation.
By using a starfish oocytes bioassay method, we have found some bioactive marine natural products from marine sponges, including 11 meroditerpenoids isolated from Strongylophora strongylata. and 11 polybrominated diphenyl ethers from Phyllospongia dendyi. (Liu et al., Citation2004Citation2005). The compounds discovered by this method showed various biological activities, such as cytotoxicity, inhibition of the tubulin polymerization, anti-microorganism, and so on. Based on our previous results and current description, the starfish oocytes bioassay model is very suitable for screening bioactive agents. The method is inexpensive, easy, sensitive, and accurate.
Conclusions
Bioassay-guided isolation of a marine sponge collected at Palau led to the isolation of three sesquiterpene quinone metabolites. They were determined to be ilimaquinone (1), 5-epi.-ilimaquinone (2), and 5-epi.-isospongiaquinone (3) by NMR data analysis. Compounds 2 and 3 were obtained as a mixture (ca. 4:1). All isolated compounds showed cytotoxicity on human tumor cell lines of NCI-H460, HepG2, SF-268, Hek-293, and HeLa, inhibitory effects on starfish oocytes maturation, and G0/G1 cell cycle arrest on HepG2 cell line.
Acknowledgments
The authors express great appreciation to Miss Xun Zhang and Mr. Hao Gao for their kind help in measuring the NMR and MS data and to Dr. Mamoru Endo of the Marine Biotechnology Institute, Japan, for the sample collection at Palau.
References
- Aoki S, Kong DX, Matsui K, Rachmat R, Kobayashi M (2004): Sesquiterpene aminoquinones, from a marine sponge, induce erythroid differentiation in human chronic myelogenous leukemia, K562 cells. Chem Pharm Bull 52: 935–937.
- Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR (2004): Marine natural products. Nat Prod Rep 21: 1–49.
- Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR (2005): Marine natural products. Nat Prod Rep 22: 15–61.
- Capon JR, MacLeod JK (1987): Revision of the absolute stereochemistry of ilimaquinone. J Org Chem 52: 5059–5060.
- Capon, RJ (1995): In: Atta-ur Rahman, ed., Studies in Natural Products Chemistry, Structure and Chemistry (Part C)., Vol. 15. Chapter 6 Marine sesquiterpene quinines and antimicrobial activity of amphibian venoms. Elsevier, Amsterdam, pp. 289–326.
- Duckworth AR (1999): The discovery and development of marine compounds with pharmaceutical potential. J Biotech 70: 15–25.
- Evans TP, Cornell L, Peterson RW, Faulkner DJ (1994): Isolation and synthesis of a glycine derivative of ilimaquinone from Fasciospongia. sp. Nat Prod Lett 4: 287–291.
- Goclik E, Koenig GM, Wright AD, Kaminsky R (2000): Pelorol from the tropical marine sponge. Dactylospongia elegans. J Nat Prod 63: 1150–1152.
- Hirsch S, Rudi A, Kashman Y, Loya Y (1991): New avarone and avarol derivatives from the marine sponge Dysidea cinerea.. J Nat Prod 54: 92–96.
- Kondracki ML, Guyot M (1989): Biologically active quinone and hydroquinone sesquiterpenoids from the sponge Smenospongia. sp. Tetrahedron 45: 1995–2004.
- Kushlan DM, Faukner DJ, Parkanyi L, Clardy J (1989): Metabolites of the Palauan sponge Dactylospongia. sp. Tetrahedron 45: 3307–3312.
- Kwak JH, Schmitz FJ, Kelly M (2000): Sesquiterpene quinols/quinines from the Micronesian sponge Petrosaspongia metachromia.. J Nat Prod 63: 1153–1156.
- Liu HW, Namikoshi M, Meguro S, Nagai H, Kobayashi H, Yao XS (2004): Isolation and characterization of polybrominated diphenyl ethers as inhibitors of microtubule assembly from the marine sponge Phyllospongia dendyi. collected at Palau. J Nat Prod 67: 472–474.
- Liu HW., Namikoshi M, Akano K, Kobayashi H, Nagai N, Yao X. S (2005): Seven new meroditerpenoids, from the marine sponge Strongylophora strongylata., that inhibited the maturation of starfish oocytes. J Asian Nat Prod Res 7: 661–670.
- Loya S, Hizi A (1993): The interaction of illimaquinone, a selective inhibitor of the RNase H activity, with the reverse transcriptases of human immunodeficiency and murine leukemia retroviruses. J Biol Chem 268: 9323–9328.
- Loya S, Tal R, Kashman Y, Hizi A (1990): Illimaquinone, a selective inhibitor of the RNase H activity of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother 34: 2009–2012.
- Luibrand RT, Erdman TR, Vollmer JJ, Scheuer PJ, Finer J, Clardy J (1979): Ilimaquinone, a sesquiterpenoid quinone from a marine sponge. Tetrahedron 35: 609–612.
- Marinlit A (2001): A marine literature database produced and maintained by the Department of Chemistry, University of Canterbury, New Zealand. http://www.Chem.Canterbury.ac.nz/marinlit.
- McConnell OJ, Longley R, Gunasekera M (1992): Isometachromin, a new cytotoxic sesquiterpenoid from a deep water sponge of the family Spongiidae. Experientia 48: 891–892.
- Mitome H, Nagasawa T, Miyaoka H, Yamada Y, Soest RWM (2001): Dactyloquinones A and B, novel sesquterpenoid quinines from the Okinawa marine sponge, Dactylospongia elegans.. J Nat Prod 64: 1506–1508.
- Mitome H, Nagasawa T, Miyaoka H, Yamada Y, Soest RWM (2002): Dactyloquinones C, D and E novel sesquterpenoid quinines, from the Okinawa marine sponge, Dactylospongia elegans.. Tetrahedron 58: 693–1696.
- Munro MHG, Blunt JW, Dumdei EJ, Hickford SJH, Lill RE, Li S, Battershill CN, Toraya T, Maoka T, Tsuji H, Kobayashi M (1995): Purification and structural determination of an inhibitor of starfish oocyte maturation from a Bacillus species. Appl Environ Microbiol 61: 1799–1804.
- Nakamura H, Deng S, Kobayashi J, Ohizumi Y, Hirata Y (1986): Dictyoceratin-A and -B, novel antimicrobial terpenoids from the Okinawan marine sponge Hippospongia. sp. Tetrahedron 42: 4197–4201.
- Nambiar MP, Wu HC (1995): Ilimaquinone inhibits the cytotoxicities of ricin, diphtheria toxin, and other protein toxins in Vero cell. Exp Cell Res 219: 671–678.
- Pous C, Chabin K, Drechou A, Barbot L, Phung-Koskas T, Settegrana C, Bourguet-Kondracki M, Maurice M, Cassio D, Guyot M, Durand G (1998): Functional specialization of stable and dynamic microtubules in protein traffic in XWIF-B cells. J Cell Biol 142: 153–165.
- Rangel HR, Dagger F, Compagnone RS (1997): Antiproliferative effect of illimaquinone on Leishmania mexicana.. Cell Biol Int 21: 337–339.
- Rodriguez J, Quinoa E, Riguera R, Peters BM, Abrell LM, Crews P (1992): The structures and stereochemistry of cytotoxic sesquiterpene quinines from Dactylospongia elegans.. Tetrahedron 32: 6667–6680.
- Salmoun M, Devijver C, Daloze D, Braekman JC, Gomez R, Kluijver M, Van Soest RWM (2000): New sesquiterpene/quinines from two sponges of the genus Htrtios.. J Nat Prod 63: 452–456.
- Shen YC, Hsieh PW (1997): New sesquiterpene hydroquinones from a Taiwanese marine sponge. Polyfibrospongia australis.. J Nat Prod 60: 93–97.
- Takizawa PA, Yucel JK, Veit B, Faulkner DJ, Deerinck T, Soto G, Ellisman M, Malhotra V (1993): Complete vesiculation of golgi membranes and inhibition of protein transport by a novel sea sponge metabolite, ilimaquinone. Cell 73: 1079–1090.
- Talpir R, Rudi A, Kashman Y, Loya Y, Hizi A (1994): Three new sesquiterpene hydroquinones from marine origin. Tetrahedron 50: 4179–4184.
- Urban S, Capon RJ (1992): 5-epi.-Isospongiaquinone, a new sesquiterpene/quinone antibiotic from an Australian marine sponge, Spongia hispida.. J Nat Prod 55: 1638–1642.
- Utkina NK, Denisenko VA, Scholokova OV, Virovaya MV, Prokof'eva NG (2003): Cyclosmenospongine, a new sesquiterpenoid aminoquinone from an Australian marine sponge Spongia. sp. Tetrahedron 44: 101–102.
- Wang SL, Cai B, Cui CB, Liu HW, Wu CF, Yao XS (2004): Diosgenin-3-O-α-L-rhamnopyranosyl-(1 → 4)-β-D-glucopyranoside, a new anticancer agent from plant resources, induces apoptosis on human colon carcinoma HCT-15 cells via mitochondria-controlled apoptotic pathway. J Asian Nat Prod Res 6: 115–125.