Abstract
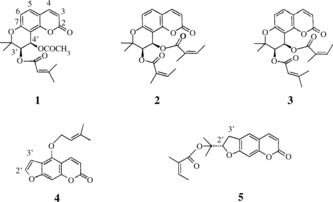
Seseli resinosum. Freyn et Sint. (Umbelliferae) is a perennial herb that grows in the northern region of Anatolia, Turkey. The n.-hexane extract obtained from the roots of S. resinosum. was investigated for the presence of coumarins. Three angular-type pyranocoumarins and two linear-type furocoumarins were isolated from the roots. The compounds were identified as (+)-samidin [(3′S., 4′S.)-3′-senecioyloxy-4′-acetoxy-3′, 4′-dihydroseselin] (1), (−)-anomalin [(3′R., 4′R.)-3′, 4′-diangeloyloxy-3′, 4′-dihydroseselin] (2), calipteryxin (3′R., 4′R.)-3′-angeloyloxy-4′-senecioyloxy-3′, 4′-dihydroseselin (3), isoimperatorin (4), and deltoin (5). The structures were determined using spectroscopic methods (1H NMR, 13C NMR, 1H-1H COSY, 1H-13C COSY, HMBC, MS), physical methods (melting point and optical rotation), and chemical correlations with known compounds that have been described in the literature. The chemotaxonomic significance of these isolated coumarins is discussed in the genus Seseli. L.
Introduction
The genus Seseli. L. is represented by 12 taxa (11 species and 1 subspecies) in the Flora of Turkey., four of which, including the title species, are native to the region. S. resinosum. Freyn et Sint. (Umbelliferae) is a perennial and endemic species that grows in northern Anatolia, Turkey (Hedge & Lamond, Citation1972; Davis et al., Citation1988; Duman, Citation2000; Parolly & Nordt, Citation2001).
The genus Seseli. is known to contain coumarins, a number of which are known for their medicinal properties. The chemical composition of S. resinosum. has not been investigated thus far, with the exception of an investigation of the essential oil composition of the fruit (Do an et al., Citation2006). Furocoumarins and pyranocoumarins are well defined groups in the genera of the Umbelliferae family. Furocoumarins are used therapeutically for the treatment of vitiligo and psoriasis; angular-type dihydropyranocoumarins in plants show antagonistic activity for calcium (Asahara et al., Citation1984; Kong et al., Citation1996).
In our previous studies, we described a simple coumarin and five angular-type pyranocoumarins, one of which is a new angular-type pyranocoumarin, corymbocoumarin, isolated from the aerial parts of S. gummiferum. subsp. corymbosum., which is also an endemic species to Turkey (Tosun, Citation2002; Tosun et al., Citation2003Citation2005a).The essential oils of some Turkish Seseli. species were investigated in previous studies (Tosun et al., Citation2005b; Do an et al., Citation2006; Tosun et al., in press). Recently, antimicrobial (Tosun et al., Citation2004), anti-inflammatory, and antinociceptive activities (Küpeli et al., in press) were examined in Turkish Seseli. species.
Herein, we report the isolation of the coumarin constituents from the n-.hexane extract of the roots of S. resinosum.; three angular-type pyranocoumarins (1–3) and two linear-type furocoumarins (4, 5). To the best of our knowledge, this is the first report of coumarins from S. resinosum., a Seseli. species endemic to Turkey. In addition, the chemotaxonomic significance of these couzmarins was evaluated and compared with Seseli. L. species, as well as other Umbelliferae and Rutaceae plants containing coumarin-type compounds.
Materials and Methods
General experimental procedures
Column chromatography (CC) was carried out on silica gel (0.063–0.200 mm, 70-230 mesh Merck 1.07734) and thin-layer chromatography (TLC) on silica gel 60 F254 (Merck 1.05554). The spots were observed under UV light at 366 nm and 254 nm, and the TLC plate was then sprayed with sulfuric acid and 5% KOH solution. Melting points were determined on a Buchi SMP-20 and an electrothermal melting point apparatus. Optical rotations were measured on a JASCO DIP-140 automatic polarimeter at 20°C. 1H and 13C NMR spectra were recorded using a JEOL JNM-EX 270 and 400 FT-NMR spectrometer in CDCl3. Chemical shifts are expressed in δ. units relative to TMS (δ. = 0) as internal standard. COSY and HMBC were also carried out using the same spectrometer. Mass spectra were obtained using a JEOL-JMS DX 302 MS spectrometer operating at 70 eV in electron impact mode.
Plant material
Seseli resinosum. was collected from Çakraz-Bartin in July 2000 at an altitude of 0–5 m during the flowering season. A voucher specimen was identified by Prof. Hayri Duman at Gazi University, Faculty of Science and Letters, and deposited at the herbarium of the Faculty of Pharmacy at Ankara University (AEF 21696).
Extraction and isolation of coumarins
The dried roots of Seseli resinosum. (600 g) were pulverized and extracted under reflux with n-.hexane, EtOAc, and MeOH, respectively. The extracts were evaporated to dryness under vacuum. The n.-hexane extract (23.86 g) was subjected to silica gel column chromatography eluting with n.-hexane/EtOAc/MeOH, with increasing polarity to yield 195 fractions. The fraction eluted with n-.hexane:EtOAc (95:5) gave compound 4 (20 mg), and elution with n-.hexane:EtOAc (80:20) yielded compounds 1 (274 mg), 2 (156 mg), 3 (80 mg), and 5 (45 mg) ().
Compound 1
(3′S., 4′S.)-3′-Senecioiloxy-4′-acetoxy-3′, 4′-dihydroseselin, (+)-(samidin), colorless needless, [α.]D20 = + 13.8° (EtOH, c 1.0), mp. 131.3–134.3°C, EIMS: C21H22O7, M+, m./z. (%): 386 (20.9), 286 (15.8), 244 (46.8), 229 (95.5), 191 (15.5), 83 (base peak, 100), 55 (11.1), 1H NMR (270 MHz, in CDCl3, δ. ppm, see ), 13C NMR (see ).
Table 1.. 1H NMR spectral data of pyranocoumarins (1–3) (ppm from TMS in CDCl3).
Table 2.. 13C NMR spectral data of pyranocoumarins (1–3) (ppm from TMS in CDCl3).
Compound 2
(−)-(3′R., 4′R.)-3′, 4′-Diangeloiloxy-3′, 4′-dihiydroseselin, (−)-anomalin, colorless needles, [α.]D20 = − 45.88° (CHCl3, c. 1.0), mp. 179.0–182.2°C, EIMS: C24H26O7, M+, m./z. (%): 426 (1.9), 328 (9.5), 327 (46.4), 326 (22.8), 311 (31.6), 243 (9.9), 229 (39.5), 227 (14.5), 213 (10.3), 83 (base peak, 100), 55 (31.2), 1H NMR (270 MHz, in CDCl3, δ. ppm, see ), 13C NMR (see ).
Compound 3
(3′R., 4′R.)-3′-Angeloiloxy-4′-senecioiloxy-3′, 4′-dihydroseselin (calipteryxin), [α.]D20 = − 20.84° (CHCl3, c.1.0), mp. 147.0–150.2°C, EIMS: C24H26O7, M+, m/z. (%): 426 (2.8), 327 (12.0), 326 (33.9), 311 (33.6), 244 (17.7), 243 (11.4), 230 (8.6), 229 (61.5), 213 (9.5), 83 (base peak, 100), 55 (21.5), 1H NMR (270 MHz, in CDCl3, δ. ppm, see ), 13C NMR (see ).
Compound 4
Isoimperatorin, mp. 108.3–109.3°C, EIMS: C16H14O4, M+, m/z. (%): 270 (2.9), 202 (base peak, 100), 174 (16.8), 145 (6.14), 89 (4.5), 69 (25.1), 41 (8.1), 1H NMR (270 MHz, in CDCl3, δ. ppm, see ), 13C NMR (see ).
Table 3.. 1H NMR spectral data of furocoumarins 4 and 5 (ppm from TMS in CDCl3).
Table 4.. 13C NMR spectral data of furocoumarins 4 and 5 (ppm from TMS in CDCl3).
Compound 5
Deltoin, [α.]D20 = −41.42° (CHCl3, c.1.0), m.p. 81.4–83.1°C, EIMS: C19H20O5, M+, m/z. (%): 328 (8.5), 228 (41.8), 213 (base peak, 100), 187 (7.4), 83 (17.9), 55 (9.8), 1H NMR (270 MHz, in CDCl3, δ. ppm, see ), 13C NMR (see ).
The 1H NMR spectral data of the pyranocoumarins in CDCl3 (1–3; as shown in ) reveals two significant pairs of doublets at δ. 6.21–7.61 (J. = 9.24–9.57 Hz) for H-3 and H-4, and at δ. 6.80–7.38 (J. = 9.24–9.57 Hz) for H-5 and H-6 (J. = 8.58–8.79 Hz), as shown in . The doublet at δ. 5.35–5.44 (J. = 4.62–4.94 Hz) was assigned to H-3′ and that at δ. 6.57–6.70 (J. = 4.88–4.95 Hz) was assigned to 4′. From the coupling constant (J. = 4.62–4.95 Hz) for 3′ and 4′ protons were indicated to cis.-form (Okuyama & Shibata, Citation1981).
All carbons shifts for the pyranocoumarins (1–3) were easily assigned by comparison with the corresponding references (Bohlmann et al., Citation1968; Gonzales et al., Citation1979; Shibata & Okuyama, Citation1989; Takata et al., Citation1990; Ikeshiro et al., Citation1992; Padha & Goswami, Citation1995; Sarker et al., Citation1995; Fan et al., Citation2000; Kong & Zhi, Citation2003) ().
The 1H NMR spectra of coumarins 4 and 5 are characteristic for the furanocoumarin skeleton; the singlet at δ. 7.15 was attributed to C-8 of 4, each singlet at δ. 6.72 was attributed to C-5, and that at δ. 7.22 was attributed to C-8 of 5, as shown in , and each carbon signal also assigned to that of the corresponding references as shown in (Elgamal et al., Citation1979; Asahara et al., Citation1984; Grande et al., Citation1986; Fang et al., Citation1995; Chen et al., Citation1996; Wang et al., Citation1999; Jimenez et al., Citation2000; Yang et al., Citation2000; Liu et al., Citation2004).
Results and Discussion
The genus Seseli. is a very rich source of coumarins, some of which are known for their medicinal properties (Bellino et al., Citation1986; Baytop, Citation1994Citation1999). In previous work, a simple coumarin, osthol, and five angular-type pyranocoumarins, including a new coumarin, corymbocoumarin, were isolated from S. gummiferum. subsp. corymbosum., an endemic species to Turkey (Tosun et al., Citation2003Citation2005a).
In the current study, five coumarins (1–5) reported for the first time in Seseli resinosum., were isolated from the n.-hexane extract using open column chromatography. Identification of the compounds was established using NMR spectrometry and confirmed by physical measurements including melting point determination and optical rotation. Thus, compound 1 was identified as (+)-samidin [(3′S, 4′S)-3′-senecioiloxy-4′-acetoxy-3′, 4′-dihydroseselin], which has been isolated from Seseli libanotis. subsp. eu-libanotis. (Lemmich et al., Citation1966; Barrero et al., Citation1990), S. unicaule. (Barrero et al., Citation1990), and Peucedanum japonicum. (Ikeshiro et al., Citation1992; Fan et al., Citation2000). Compound 2 was determined to be (−)-anomalin [(3′R., 4′R.)-3′,4′-diangeloiloxy-dihiydroseselin], which was previously isolated from Seseli bocconi. (Bellino et al., Citation1986; Barrero et al., Citation1990). According to literature data, anomalin was isolated from a number of Seseli. species including: S. seravschanicum., S. tschuense., S. coronatum., S. asperulum., S. talassicum., S. dichotomum., S. petraeum., S. ponticum., S. tenuisectum., S. eriocephalum., and S. incanum. (Barrero et al., Citation1990). However, anomalin has been isolated from Seseli bocconi.. In addition, anomalin has been isolated from the other Umbelliferae species: Peucedanum japonicum. (Ikeshiro et al., Citation1992; Fan et al., Citation2000), Peucedanum wulongense. (Kong & Zhi, Citation2003), Ligusticum elatum. (Padha & Goswami, Citation1995), and Musenion divaricatum. (Swager & Cardellina, Citation1985). Compound 3 was identified as calipteryxin [(3′R., 4′R.)-3′-angeloiloxy-4′-senecioiloxy-3′, 4′-dihiydroseselin], which was previously isolated from an Umbelliferae plant, Musenion divaricatum. (Swager & Cardellina, Citation1985). Compound 4 was identified as isoimperatorin, and has been reported in several species including Seseli elatum. (Coassini Lokar & Delben, Citation1988; Barrero et al., Citation1990), S. rigidum., S. campestre., S. krylovii., S. talassicum., S. abolini., S. gracile. (Barrero et al., Citation1990), Angelica dahurica. (Liu et al., Citation2004; Piao et al., Citation2004; Thanh et al., Citation2004), Cachrys sicula. (Grande et al., Citation1986), Ferulago. species (Jimenez et al., Citation2000), Notopterygium forbesii. (Yang et al., Citation2000), Peucedanum japonicum. (Chen et al., 1986), Peucedanum wulongense. (Kong & Zhi, Citation2003), Dorstenia gigas. (Franke et al., Citation2001), and Leptothyrsa sprucei. (Li et al., Citation2001). Compound 5 was also found in S. campestre., S. peucedanoides., S. gracile., S. tortuosum., S. asperulum. (Barrero et al., Citation1990), Peucedanum japonicum.. (Chen et al., 1986), and Saposhnikovia divaricata. (Wang et al., Citation1999). The structure of compound 5 was established as deltoin using spectroscopic analysis. The spectral data are in good agreement with those of the literature (Elgamal et al., Citation1979; Asahara et al., Citation1984; Grande et al., Citation1986; Fang et al., Citation1995; Sarker et al., Citation1995; Chen et al., Citation1996; Bissoue et al., Citation1996; Jimenez et al., Citation2000). Further, the spectroscopic data for all of the compounds are in agreement with those of the corresponding literature data. The assignments for the 1H and 13C chemical shifts of coumarins 1–5 were supported by COSY, HETCOR, and HMBC experiments. The NMR spectral data for coumarins 1–3 are shown in Tables and , whereas those for coumarins 4 and 5 are given in Tables and .
This is the first report that describes the presence of these coumarins in S. resinosum., a member of the Umbelliferae family. Furthermore, the roots of Seseli resinosum. were determined to be a very rich source of both (+)-samidin (1) and (-)-anomalin (2). The coumarins 1–5 are found in some other Umbelliferae and Rutaceae species, and other Seseli. species except for 3. Therefore, coumarins 1, 2 and 4, 5 do not appear to have a significant chemotaxonomical relationship for the Seseli. species. However, calipteryxin (3) was reported in Musinion divaricatum. (Swager & Cardellina, Citation1985) as an Umbelliferae species. Until the current report, 3 had not been reported in the Seseli. species; therefore, it is of interest that 3 is found in Seseli resinosum., and has a chemotaxonomic significance for Seseli. species.
Up to now, naturally occurring coumarins have been isolated from over 800 species. Some of these coumarins show significant pharmacological activity (Murray et al., Citation1982; Asahara et al., Citation1984; Duh et al., Citation1991; Chen et al., Citation1996; O'Kennedy & Thornes, Citation1997; Matsuda et al., Citation1998Citation2000; Seo et al., 2001; Lee et al., Citation2003). Further, the coumarins reported in the current study also have a number of biological properties. Several of the known khellactone esters have been reported to exhibit antispasmodic and vasodilator activity. Samidin, a dihydropyranocoumarin derivate, is antihistaminic, calcium-blocking, and a cytotoxic natural product (Ikeshiro et al., Citation1992); anomalin protected the liver injury induced by D-galactosamine/lipopolysaccharide (LPS) in mice (Yoshikawa et al., Citation2005). Isoimperatorin, a common coumarin, showed antioxidant (Piao et al., Citation2004), and cytotoxic activity moderately (Thanh et al., Citation2004). Moreover, anticancer, antibacterial, and codeine effects of isoimperatorin were recorded in other literature (Liu et al., Citation2004). In addition, the compound inhibited cyclooxygenase and lipooxygenase pathways of arachidonate metabolism (Abad et al., Citation2001). Deltoin is a strong inhibitor in NO production of LPS activated macrophages (Wang et al., Citation1999). Therefore, the coumarins identified in this plant may be investigated for possible pharmacological activity in future work.
References
- Abad MJ, De Las Heras B, Silvan AM, Pascual R, Bermejo P, Rodriquez B, Villar AM (2001): Effects of furocoumarins from Cachrys trifida. on some macrophage functions. Pharm Pharmacol 53: 1163–1168.
- Asahara T, Sakakibara I, Okuyama T, Shibata S (1984): Studies on coumarins of a Chinese drug “Qian-Hu” V. Coumarin-glycosides from “Zi-Hua Qian-Hu.” Planta Med 18: 488–491.
- Barrero AF, Herrador MM, Arteaga PC (1990): Cumarinas en especies del genero Seseli. (Fam. Umbelliferae). Ars Pharm 31: 241–256.
- Baytop T (1994): Türkçe Bitki Adlari Sözlügü. Atatürk Kültür, Dil ve Tarih Yüksek Kurumu, TDKY 3578, Ankara, TTK Basimevi.
- Baytop T (1999): Therapy with Plants in Turkey (Past and Present), 2nd ed. Istanbul, Nobel Medical Book House.
- Bellino A, Venturella P, Marino ML, Servettaz O, Venturella G (1986): Coumarins from Seseli bocconi.. Phytochemistry 25: 1195–1199.
- Bissoue AN, Muyard F, Bevalot F, Tillequin F, Mercier M-F, Armstrong JA, Vaquette J, Waterman PG (1996): Coumarins from the aerial parts of Chorilaena quercifolia.. Phytochemistry 43: 877–879.
- Bohlmann F, Bhaskar Rao VS, Grenz M (1968): Über die cumarine aus Angėlica ursina. und Seseli libanotis.. Tetrahedron Lett 36: 3947–3950.
- Chen S, Chang CT, Sheen WS, Teng CM, Tsai IL, Duh CY, Ko FN (1996): Coumarins and antiplatelet aggregation constituents from formosan Peucedanum japonicum.. Phytochemistry 41: 525–530.
- Coassini Lokar LR, Delben S (1988): Photoactive furocoumarins in two populations of Seseli elatum.. Phytochemistry 27: 1073–1077.
- Davis PH, Mill RR, Tan K (1988): Flora of Turkey and the East Aegean Islands, vol. 10. Edinburgh, Edinburgh University Press.
- Duh C-Y, Wang S-K, Wu Y-C (1991): Cytotoxic pyranocoumarins from the aerial parts of Peucedanum japonicum.. Phytochemistry 30: 2812–2814.
- Duman H (2000): Seseli L.. In: Güner A,Özhatay N, Ekim T, Baer KHC, eds., Flora of Turkey and The East Aegean Islands, Vol. 11. Edinburgh, Edinburgh University Press.
- Do an E, Duman H, Tosun A, Kürkçüo lu M, Ba er KHC (2006): Essential oil composition of the fruits of Seseli resinosum. Freyn et Sint. and Seseli tortuosum. L. growing in Turkey. J Essent Oil Res ( in press).
- Elgamal MHA, Elewa NH, Elkhrisy EAM, Duddeck H (1979): 13C-NMR chemical shifts and carbon-proton coupling constants of some furocoumarins and furochromones. Phytochemistry 18: 139–143.
- Fan B, Baba M, Mizuno A, Okada Y, Xu J, Okuyama T (2000): Studies on the chemical constituents of Peucedanum japonicum. (Umbelliferae). J Jpn Botany 75: 257–261.
- Fang S-C, Shieh B-J, Wu R-R, Lin C-Nan (1995): Isoprenylated flavonols of formosan Broussonetia papyrifera.. Phytochemistry 38: 535–537.
- Frananke K, Porzel A, Masaoud M, Adam G, Schmidt J (2001): Furanocoumarins from Dorstenia gigas.. Phytochemistry 56: 611–621.
- Gonzales AG, Barroso JT, Dorta HL, Luis JR, Rodriguez-Luis F (1979): Pyranocoumarin derivatives from Seseli tortuosum.. Phytochemistry 18: 1021–1023.
- Grande M, Aguado MT, Mancheno B, Piera F (1986): Coumarins and ferulol esters from Cachyrs sicula.. Phytochemistry 25: 505–507.
- Hedge IC, Lamond JM (1972): Seseli. L. In: Davis PH, ed., Flora of Turkey and the East Aegean Islands, Vol. 4. Edinburgh, Edinburgh University Press, pp. 367–372.
- Ikeshiro Y, Mase I, Tomita Y (1992): Dihydropyranocoumarins from roots of Peucedanum japonicum.. Phytochemistry 31: 4303–4306.
- Jimenez B, Grande MC, Anaya J, Torres P, Grande M (2000): Coumarins from Ferulago capillaries. and F. brachyloba.. Phytochemistry 53: 1025–1031.
- Kong L-Y, Min Z-D, Li Y, Li X, Pei Y-H (1996): Qianhucoumarin I from Peucedanum japonicum.. Phytochemistry 42: 1689–1691.
- Kong LY, Zhi F (2003): Coumarins from Peucedanum wulongense.. J Asian Nat Prod Res 5: 183–187.
- Küpeli E, Tosun A, Yesilada E (2005): Anti-inflammatory and antinociceptive activities of Seseli. L. species (Apiaceae) growing in Turkey. ( in press).
- Lemmich J, Lemmich E, Nielsen BE (1966): Constituents of Umbelliferous plants. VIII. Coumarins from the root of Seseli libanotis. (L.) Koch. The structure of new coumarins. Acta Chem Scand 20: 2497–2507.
- Lee YY, Lee S, Jin JL, Yun-Choi HS (2003): Platelet anti-aggregatory effects of coumarins from the roots of Angelica genuflexa. and A. gigas.. Arch Pharm Res 26: 723–726.
- Li X, Dunbar DC, ElSohyl HN, Walker LA, Clark AM (2001): Indolopyrido-quinazoline alkaloid from Leptothyrsa sprucei.. Phytochemistry 58: 627–629.
- Liu R, Li A, Sun A (2004): Preparative isolation and purification of coumarins from Angelica dahurica. (Fisch. ex Hoffm) Benth. et Hook. F (Chinese traditional medicinal herb) by high-speed counter-current chromatography. J Chromatogr A 1052: 223–227.
- Matsuda H, Murakami T, Kageura T, Ninomiya K, Toguchida I, Nishida N, Yoshikawa M. (1998): Hepatoprotective and nitric oxide production inhibitory activities of coumarin and polyacetylene constituents from the roots of Angelica furcijuga.. Bioorg Med Chem Lett 8: 2191–2196.
- Matsuda H, Murakami T, Nishida N, Kageura T, Ninomiya K, Yoshikawa M (2000): Vasorelaxant active constituents from the roots of Angelica furcijuga. KITAGAWA: Structures of hyuganins A, B, C, and D. Chem Pharm Bull 48: 1429–1435.
- Murray RDH, Mendez J, Brown SA (1982): The Natural Coumarins. New York, John Wiley & Sons Ltd.
- O'Kennedy R, Thornes RD (1997): Coumarins: Biology, Applications and Mode of Action. Chichester, John Wiley & Sons.
- Okuyama T, Shibata S (1981): Studies on coumarins of a Chinese drug Qian-Hu. Planta Med 42: 89–96.
- Padha N, Goswami KN (1995): Anomalin. A dihydropyranocoumarin derivative from the plant Ligusticum elatum.. Acta Cryst 51: 2710–2712.
- Parolly G, Nordt B (2001): Seseli hartvigii. (Apiaceae) a new name for S. ramosissimum. Hartvig & Strid, with carpological and ecological notes on this species. Willdonowia 31: 87–93.
- Piao XL, Park HI, Baek SH, Kim HY, Park MK, Park JH (2004): Antioxidative activity of furanocoumarins isolated from Angelica dahurica.. J Ethnopharmacol 93: 243–246.
- Sarker SD, Armstrong JA, Waterman PG (1995): An alkaloid, coumarins and a triterpene from Boronia algida.. Phytochemistry 39: 801–804.
- Seo E-K, Kim KH, Kim MK, Cho M-H, Choi EW, Kim KN, Mar W (2002): Inhibitors of 5α.-reductase type I in LNCaP cells from the roots of Angelica koreana.. Planta Med 68: 162–163.
- Shibata S, Okuyama T (1989): Chemistry and pharmacology of Qianhu. Abstracts of Chinese Medicines 3: 214–230.
- Swager TM, Cardellina JH (1985): Cumarins from Musenion divaricatum.. Phytochemistry 24: 805–813.
- Takata M, Shibata S, Okuyama T (1990): Structures of angular pyranocoumarins of Bai-Hua Qian-Hu, the root of Peucedanum praeruptorum.. Planta Med 56: 307–311.
- Thanh PN, Jin WY, Song GY, Bae K, Kang SS (2004): Cytotoxic coumarins from the root of Angelica dahurica.. Arch Pharm Res 27: 1211–1215.
- Tosun A, Baba M, Özkal N, Okuyama T (2003). Coumarins from Seseli gummiferum. Pall. ex. Sm. subsp. corymbosum. P.H. Davis. Natural Medicines 57: 117
- Tosun A, Özkal N, Y1ld1z S (2004): Antimicrobial activity screening of some Seseli. L. species growing in Turkey. J Fac Pharm Ankara 33: 151–155.
- Tosun A, Özkal N, Baba M, Okuyama T (2005a): Pyranocoumarins from Seseli gummiferum. subsp. corymbosum. growing in Turkey. Turkish J Chem 29: 327–334.
- Tosun A, Baba M, Kodama T, Nakanishi H, Okuyama T (2005b): The composition of essential oil of Seseli. L. species growing in Turkey. Natural Medicines 59: 85–90.
- Tosun A, Kürkçüo lu M, Do an E, Duman H, Ba er KHC (2006): Essential oil composition of Seseli petraeum. M. Bieb. and Seseli andronakii. Woron. growing in Turkey. Flav Frag J ( in press).
- Tosun A (2002): Pharmacognostical researches on some endemic Seseli. L. species growing in Turkey. PhD Thesis, Ankara, Turkey.
- Wang C-C, Chen L-G, Yang L-L (1999): Inducible nitric oxide synthase inhibitor of the Chinese herb I. Saposhnikovia divaricata. (Turcz.) Schischk. Cancer Lett 145: 151–157.
- Yang F, Zhang T, Liu Q, Xu G, Zhang Y, Zhang S, Ito Y (2000): Preparative isolation and purification of notopterol and isoimperatorin from Notopterygium forbessi. Boiss (Chinese traditional medicinal herb) by high-speed counter-current chromatography. J Chromatogr A 883: 67–73.
- Yoshikawa M, Nishida N, Ninomiya K, Ohgushi T, Kubo M, Morikawa T, Matsuda H (2005): Inhibitory effects of coumarin and acetylene constituents from the roots of Angelica furcijuga. on D-galactosamine/lipopolysaccharide-induced liver injury in mice and on nitric oxide production in lipopolysaccharide-activated mouse peritoneal macrophages. Bioorg Med Chem ( in press).