Abstract
In Mexican traditional medicine, Bocconia frutescens. L. (Papaveraceae) is known as “gordolobo” or “llorasangre.” Natives use this plant to treat skin ulcer, dermatitis, and some respiratory tract infections. In this study, the aerial parts afforded the alkaloids dihydrochelerythrine, (±)-6-acetonyldihydrochelerythrine, (±)-6-acetonyldihydrosanguinarine, as well as β.-amyrine acetate and 2-decanol. The structures were determined by spectral methods including UV, IR, NMR and mass spectrometry. The antimicrobial activity of extracts and pure compounds was tested with Escherichia coli., Staphylococcus aureus., Pseudomonas aeruginosa., and Bacillus subtilis.. This study supports the use of B. frutescens. in traditional medicine.
Introduction
Use of plants by human societies has a long and interesting history. It is well-known that, since remote times, plants satisfy diverse necessities, among which are recovery and maintenance of health; this is very important in both rural and urban areas in many countries. The knowledge of the local flora by numerous ethnic groups of México represents one of the main linkages to their past culture. An important part of this flora is traditionally used by villagers as medicinal plants for alleviating a large number of common diseases. This is also true for many ethnic cultures of other countries.
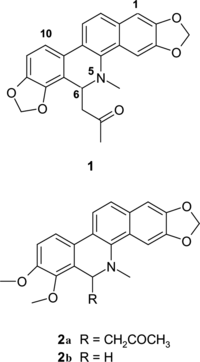
Various studies involving chemistry and biological activities have been undertaken on species of the genus Bocconia. (Papaveraceae). From Bocconia latisepala. were isolated the compounds sanguinarine, protopine, chelerythrine, and allocryptopine (Domínguez, Citation1965), which were also isolated from B. arborea. and B. cordata. (McLean et al., Citation1969). From B. cordata., Konda et al. (Citation1985) isolated, in addition, the compound bocconine. From the lipophilic leaf extract of B. integrifolia. was identified the compound 12-methoxydihydrochelerythrine (Oechslin et al., Citation1991). Navarro et al. detected antimicrobial compounds in B. arborea. (Navarro et al., Citation1998). Recently isolated from aerial parts of Bocconia arborea. were the compounds (±)-6-acetonyldihydrosanguinarine (1), (±)-6-acetonyldihydrochelerythrine (2a) (), (±)-6-methoxydihydrochelerythrine, (±)-sanguidimerine, chelidimerine, and two new compounds named bocconarborine A and B (Julián & Delgado, Citation2001). From the roots of Bocconia frutescens., bioactive benzo(c.)phenanthridine alkaloids were isolated, which inhibit specific receptors of the human angiotensin AT1 and endothelin ETA (Caballero-George et al., Citation2002Citation2003).
This is the first time that the phytochemistry of the aerial parts of Bocconia frutescens. L. and the biological activity of their extracts were studied in order to support its use in traditional medicine. The aerial parts of this plant are used in an infusion or pulverized. The infusion is used to cure some digestive and respiratory tract infections such as bronchitis (Martínez, Citation1984). On the other hand, the juice of this plant or the pulverized plant is used in the treatment of skin ulcers and eruptions (Martínez, Citation1977). In México, B. frutescens., traditionally known as “gordolobo” or “llorasangre,” is a tree that grows in the Tamaulipas, San Luis Potosí, Veracruz, Oaxaca, Chiapas, and Puebla states.
Materials and Methods
General experimental procedures
Column chromatography was carried out on Macherey-Nagel Silica gel 60 (70-230 mesh). Optical rotations were measured on a Perkin-Elmer 341 polarimeter (Germany). UV spectra were measured on a Varian Cary 100 spectrometer (Australia). IR spectra were obtained on a Biorad FTS135 spectrometer (USA), and 200 MHz 1H and 50.3 MHz 13C NMR spectra were recorded on a Varian Gemini 2000 spectrometer (USA).
Plant material
This plant was collected in the Veracruz state, México, during December 2001. A specimen voucher (V-5488) was deposited at the herbarium of the Instituto de Investigaciones Biológicas, Universidad Veracruzana, Xalapa region.
Preliminary tests
Qualitative tests for leaves and stems, involving coloration and precipitation reactions, were performed to detect active principles like alkaloids, leukoanthocyanins, saponins, triterpenes, glycosides, steroids, and anthraquinones. These tests were performed according to the method described by Domínguez (Citation1973).
Extraction and isolation
Dry and pulverized leaves (200 g) of B. frutescens. were extracted with hexane to afford green oil (15 g). A portion (5 g) was column chromatographed on silica gel. Fractions eluted with hexane-CH2Cl2-ethanol (1:1:0.1) contained (±)-6-acetonyldihydrochelerythrine (2a) as a yellowish solid, which was recrystallized to obtain fine and colorless crystals; the next fractions, eluted with the same solvent, contained the mixture of (2a) and dihydrochelerythrine (2b) (), which was further purified in a silica gel column using CHCl3-methanol.
On the other hand, dry leaves of B. frutescens. (235 g) were extracted with ethanol to afford green oil (16 g). A portion (8 g) was also column chromatographed on silica gel using hexane-EtOAc (0–100%) as solvent. From the first fractions, eluted with hexane, 2-decanol was isolated. Fractions eluted with hexane-EtOAc (19:1) contained (±)-6-acetonyldihydrosanguinarine (1), which was purified by other column chromatography. Fractions eluted with hexane-EtOAc (9:1) contained β.-amyrine acetate, which was purified by crystallization (Mahato & Kundu, Citation1994).
Biological method of evaluation
Disk diffusion method, employed to evaluate the extracts, is one of the bioassays mostly utilized for its simplicity and reproducibility in Müeller-Hinton [National Committee for Clinical Laboratory Standards: Performance standards for antimicrobial disk susceptibility test (Approved standard, M2-A5). Villanova, PA, USA, National Committee for Clinical Laboratory Standards, 1993]. The microorganisms used as reference for this test were Staphylococcus aureus. (ATCC 4012), Bacillus subtilis. (ATCC 465), Pseudomonas aeruginosa. (ATCC 260), and Escherichia coli. (ATCC 128). Positive control was ciprofloxacin (5 µg/disk). Negative control was sterile distilled water.
Microorganisms were inoculated in 5 ml of Trypticase Soy Broth (TSB) and incubated during 18 h at 37°C. Then, the inoculums were adjusted in comparison with Tube 0.5 of McFarland standard (equivalent to 1.5 × 106 units of colonies formed per milliliter). The extracts to test were prepared in a solution at 5% with the proper solvent. The paper filter disks were sterilized and impregnated with 10 µl of samples, previously filtered through a 0.22-µm filter. The negative and positive controls were prepared as described for the samples at the same time.
Each strain was spread with a cotton swab in three directions. After the plates were dried, the disks impregnated with each extract were placed over the agar. Each test was made by three replications and then incubated for 24 h at 37°C. The positive cases were determined by the presence of an inhibition zone in the disk containing the extract, which is measured with the aid of a ruler or vernier.
Results and Discussion
Preliminary tests for detection of secondary metabolites
These tests were performed in the laboratory following the methodology mentioned above. In is listed the results for leaves and stems.
Table 1.. Metabolites in leaves and stems of Bocconia frutescens..
Identification of secondary metabolites
In this study, the following substances were isolated: (±)-6-acetonyldihydrosanguinarine (1), (±)-6-acetonyldihydrochelerythrine (2a), dihydrochelerythrine (2b), β.-amyrine acetate, and 2-decanol. The compounds isolated were purified by column chromatography on silica gel. The structures were determined by UV, IR, 1H and 13C NMR, and mass spectra. These spectra are in good agreement with the spectral data reported in the literature (Mahato & Kundu, Citation1994; Julián & Delgado, Citation2001).
Antimicrobial activity
The results of the bioassays of antimicrobial activity for E. coli., S. aureus., P. aeruginosa., and B. subtilis. are summarized in , in which is shown the activity of the whole extracts against E. coli. and S. aureus..
Table 2.. Inhibition halos of B. frutescens. extracts.
The diffusion test in disk was undertaken for the four purified compounds isolated from the leaves of B. frutescens. to detect possible antibacterial activity, but none of the pure compounds showed inhibitory effects on bacteria.
Conclusions
B. frutescens. is distributed widely in tropical and temperate zones, pine and mesophyllic forest. In the America continent, its distribution goes from north of México to south of Bolivia, including the Caribbean islands. Common names in México, among others, are “cuatlataya,” “gordolobo,” “llora sangre,” “mano de león,” and “palo santo.”
Because of the different kinds of compounds observed in preliminary phytochemical tests like alkaloids, saponins, terpenes, and steroids, the medicinal value of this species was established. It is already established that antibacterial action is attributed to the presence of these metabolites. Hence, detection of these kinds of metabolites is very important to decide to test the extracts for antimicrobial effectiveness.
With regard to the antimicrobial activity, the inhibition grade presented by different extracts on the bacterial cultures clearly showed the action of leaf ethanol extract as well as the stem hexane extract on S. aureus. (Gram positive) and E. coli. (Gram negative) ().
Both leaf hexane extract and stem CH2Cl2 extract showed moderate activity. However, the concentration was not sufficient to cause an evident activity on P. aeruginosa. and B. subtilis. cultures; only the positive control showed activity against these bacteria.
Because more than 90% of Staphylococcus aureus. strains are resistant to penicillin and other antibiotics (Coello et al., Citation1994) and may cause important diseases, it is very significant that extracts of Bocconia frutescens. show activity against this microorganism. S. aureus. may mainly cause infections in the respiratory tract and in skin wounds. On the other hand, E. coli. may cause infections in the digestive tract. Based on the fact that the extracts of Bocconia frutescens. show activity against these microorganisms, its traditional medicine use, like those described in the “Introduction” section, is totally supported.
The chemical study on leaf ethanol and hexane extracts afforded (±)-6-acetonyldihydrochelerythrine (2a), (±)-6-acetonyldihydrosanguinarine (1), β.-amyrine acetate, and 2-decanol, but no compound showed activity with the bacteria used. This fact can be due to the following factors: (a) the activity is due to a synergic action; (b) the active substance is a product of the metabolic transformation of the isolated products; (c) the isolated metabolites really have no activity against the bacteria used. More studies should be performed to elucidate these suggestions.
This plant is used in many countries of the Americans as a medicinal; however, in other countries of the world, this species is considered as a pest. For example, in Hawaii, where this plant is known as plume poppy, it is being fought due to its invasive ability (ISCs & CGAPS, Citation2003; Chimera, Citation2004; Klasner et al., Citation2004). This plant was first cultivated as ornamental, but now grows wild. No medicinal use is given in Hawaii; however, in light of these investigations, it would be very important and useful to take advantage of this plant as a medicinal, instead of eradicating it from Hawaii's forests.
Acknowledgment
We wish to thank to M.S. Santiago Mario Vázquez Torres for plant collection and its classification.
References
- Caballero-George C, Vanderheyden PML, Apers S, Van den Heuvel H, Solis PN, Gupta MP, Claeys M, Pieters L, Vauquelin G, Vlietinck AJ (2002): Inhibitory activity on binding of specific ligands to the human angiotensin II AT1 and endothelin 1 ETA receptors: Bioactive benzo[c]phenanthridine alkaloids from the root of Bocconia frutescens.. Planta Med 68: 770–775.
- Caballero-George C, Vanderheyden PML, Solis PN, Gupta MP, Pieters L, Vauquelin G, Vlietinck AJ (2003): In vitro. effect of sanguinarine alkaloid on binding of [3H]candesartan to the human angiotensin AT1 receptor. Eur J Pharmacol 458: 257–262.
- Chimera C (2004): Bird-facilitated dispersal of the invasive tree Bocconia (Bocconia frutescens.) in a Hawaiian dry forest. Hawaii Conservation Conference Abstracts p. 5. Coordinating Group on Alien Pest Species (CGAPS). Honolulu, HI.
- Coello R, Jiménez J, García M, Arroyo P, Minguez D, Fernández C, Cruzet F, Gaspar C (1994): Prospective study of infection, colonization and carriage of methicillin-resistant Staphylococcus aureus. in an outbreak affecting 990 patients. Eur J Clin Microbiol Infect Dis 13: 74–81.
- Domínguez XA (1973): Métodos de Investigación Fitoquímica. México, Noriega Editores, Editorial LIMUSA.
- Domínguez XA, García J, Monroy A (1965): A chemical study of Bocconia latisepala. Wats. Can J Chem 43: 679–682.
- ISCs and CGAPS (Invasive Species Committees of Hawaii and Coordinating Group on Alien Pest Species) (2003): Report for the Calendar 2003 year.
- Julián A, Delgado G (2001): (±)-Bocconarborines A and B, novel 1,3-bis.-benzo[c.]phenanthridinyl acetone alkaloids from Bocconia arborea.. Rev Soc Quím Méx 45: 189–194.
- Klasner F, Hu D, Dicus G (2004): National Park service invasive species inventories and “vital signs” monitoring. Hawaii Conservation Conference, Abstracts, p. 15. Coordinating Group on Alien Pest Species (CGAPS). Honolulu, HI.
- Konda Y, Harigaya Y, Onda M (1985): Studies on the constituents of Bocconia cordata.. Structure elucidation of bocconine by means of nuclear magnetic resonance spectroscopic studies. J Heterocycl Chem 23: 877–879.
- Mahato SB, Kundu AP (1994): 13C NMR spectra of pentacyclic triterpenoids—A compilation and some salient features. Phytochemistry 37: 1517–1575.
- Martínez OE (1977): Flora de Veracruz., fascículo 77. Veracruz, Instituto de Ecología, A. C. Xalapa, Riverside, CA, University of California, pp. 25–27.
- Martínez M (1984): In: Las Plantas Medicinales De México, 3rd ed. Bota: Mexico city, MX, México.
- McLean DB, Gracey DE, Saunders JK, Rodrigo R, Manske RHF (1969): Some benzophenanthridine alkaloids from Bocconia arborea.. Can J Chem 29: 1951–1956.
- Navarro V, Rojas G, Delgado G, Lozoya X (1998): Antimicrobial compounds detected in Bocconia arborea. extracts by a direct bioautographic method. Arch Med Res 29: 191–194.
- Oechslin SM, Oechslinmerkel K, Wright AD (1991): A NMR-study of 4-benzophenanthridine alkaloids. J Nat Prod 54: 519–524.