Abstract
One new prenylated xanthone, 1,5-dihydroxy-3-methoxy-4-isoprenylxanthone (1), along with four previously known prenylated xanthones, ananixanthone (2), 1,3,7-trihydroxy-2,4-diisoprenylaxnthone (3), 8-desoxygartanin (4), and toxyloxanthone A (5), have been isolated from Chrysochlamys tenuis. (Hammel) (Clusiaceae). Compound 1 showed moderate activity (31 ± 9 µM) against a chloroquine-resistant strain of Plasmodium falciparum., and compounds 3 and 5 showed the highest antimalarial potency, IC50 = 20 ± 2 and 16 ± 4 µM, respectively. Evaluated against Trypanosoma cruzi., compound 1 presented negligible activity, but compounds 2, 3, and 4 showed mild antitrypanosomal activity with IC50 values of 23 ± 4, 21 ± 5, and 24 ± 3 µM, respectively. All structures were elucidated by NMR spectroscopy in combination with UV, IR, and MS spectral data.
Introduction
Malaria kills between 1 million and 2 million people annually. Each year, 300–500 million new clinical cases are announced, and half of humanity is at risk. The cornerstone of malaria control worldwide remains effective and inexpensive drugs, but the growing resistance to existing treatments such as chloroquine creates an urgent need to discover new medicines. Malaria is one of the tropical diseases of greatest impact on world health (Gelb & Hol, Citation2002; Ridley, Citation2002). Trypanosoma cruzi. is the causative agent of Chagas disease, a parasitic disease that outranks the combined burden of malaria, schistosomiasis, and leishmaniasis in terms of its economic impact in Latin America (World Bank, Citation1993). Current estimates from the World Health Organization indicate that 16–18 million people are infected, with an additional 100 million at risk (Ortega-Barría, Citation2003).
This report presents the isolation of five xanthones from Chrysochlamys tenuis. (Clusiaceae) (Hammel), as part of the ongoing International Cooperative Biodiversity Group (ICBG) program based in Panama (Coley et al., Citation2003). The crude extract of Chrysochlamys tenuis. showed antimalarial activity (IC50 28 µg/ml); however, the MeOH and hexane soluble fractions showed the highest antimalarial activity (IC50 values of 14 and 26 µg/ml, respectively). From the MeOH fraction, five xanthones were isolated. The antifungal and antibacterial activity of naturally occurring xanthones have been known since the 1980s (Sultanbawa, Citation1980) and the “xanthone hypothesis” for antimalarial activity was put forward from the in vitro. production of a pentahydroxyxanthone (Winter et al., Citation1996). Subsequent reports indicate that xanthones present different levels of activity against multidrug-resistant Plasmodium. parasites (Ignatushchenko et al., Citation1997Citation2000). In addition, this report presents the activity of xanthones in Trypanosoma cruzi. (Chagas disease).
Materials and Methods
General experimental procedures
Melting points were determined with an Electrothermal 9100 melting point apparatus (Labequip, Ontario, Canada) and are uncorrected. The UV spectra were run on a Spectronic Genesys 2 spectrophotometer. (Milton Roy, USA) IR spectra were obtained on a Perkin-Elmer FTIR-1000 spectrophotometer. The NMR spectra were recorded on a Bruker Avance 300 MHz spectrometer (75 MHz for carbon) with TMS as an internal standard. High Resolution Chemical Ionization Mass Spectrometry (HRCIMS) were recorded on a Kratos MS50TC instrument.
Plant material
Both mature and young leaves of Chrysochlamys tenuis. were collected in Parque Nacional Altos de Campana in the Republic of Panama in May 2002 and January 2003. The species was identified by Professor Mireya Correa of the University of Panama and the Smithsonian Tropical Research Institute. Vouchers of the plants were deposited in the herbarium of the University of Panama (PMA 52655).
Extraction and isolation
Upon collection, leaves were transferred to sealed plastic bags, kept on ice, and processed within 6 h. After removal of the stems, 0.25 kg of fresh mature and young leaves were homogenized and concentrated (Montenegro et al., Citation2003; Torres et al., Citation2003). Analysis by TLC on silica gel (Merck type 60 F254), hexane/CH2Cl2/acetone 6:2:2 and 4:3:3, with detection with molybdenum-H2SO4 reagent followed by heating, gave the same patterns for the MeOH and hexane fractions for both mature and young leaves. An aliquot of 6.0 g of the MeOH fraction was chromatographed on a normal phase column (Merck SI 100, 300 g; 5 × 32.5 cm), eluted sequentially with hexane/CH2Cl2/acetone (80:10:10, 800 ml), hexane/CH2Cl2/acetone (70:15:15, 600 ml), hexane/CH2Cl2/acetone (60:20:20, 600 ml), and MeOH (100%, 300 ml). The fractions were combined according to their TLC profiles into six fractions (1 to 6). Fraction 2 (1.27 g, 21.2%), eluted between 340 and 500 ml, yielded 1 (179 mg, 14.1%) following precipitation by the addition of MeOH (Rf 1: 0.39). Fraction 3 (2.0 g, 33.4%), eluted between 500 and 1200 ml, was chromatographed on a normal phase column of silica gel (silica gel 200 g; 3 × 49 cm) (J.T. Baker type 7GF, <40 µm) eluted with CHCl3 (930 ml), followed by 100% MeOH (200 ml), and the fractions were combined according to their TLC profiles into five fractions (3a to 3e). Fraction 3b yielded 2 (14 mg, 0.7%), tR of 2: between 168 and 324 ml; Rf 2: 0.31. Fraction 3c yielded 3 (101 mg, 5.1%), tR of 3 between 60 and 230 ml, Rf 3: 0.24. Fraction 3d yielded 4 (16.5 mg, 0.82%), tR of 4 between 735 and 930 ml; Rf 4: 0.14. Fraction 1 (201 mg, 3.5%), tR of the mixture of 2 and 5 between 1 and 340 ml, was subjected to preparative TLC (Whatman, PK5F 250 µm), with hexane/acetone (80:20), yielding Fractions 1a to 1c. Fraction 1b yielded 5 (11 mg, 5.2%); Rf 5: 0.14. All eluates were checked by TLC with hexane/acetone (90:10) and detected by spraying with molybdenum-H2SO4 reagent followed by heating.
1,5-Dihydroxy-3-methoxy-4-isoprenylxanthone (1)
Colorless amorphous powder; mp 175–176°C; UV (MeOH) λmax(log ε) 370 (0.399), 319 (1.511), 244 (2.950), 211 (2.027) nm; IR (CHCl3) νmax 3510, 3200 br, 1650, 1608 cm−1; 1H NMR (300 MHz, CDCl3) δ. 12.93 (1H, s, C-1-OH); 7.75 (1H, dd, J. = 7.8 and 1.8 Hz, H-8); 7.31 (1H, dd, J. = 7.9 and 1.7 Hz, H-6); 7.24 (1H, t, J. = 7.8 Hz, H-7); 6.40 (1H, s, H-2); 5.76 (1H, br s, C-5-OH); 5.22 (1H, m, J. = 6.7 Hz, H-12); 3.95 (3H, s, H-16); 3.50 (2H, br d, J. = 6.8 Hz, H-11); 1.85 (3H, s, H-14); 1.65 (3H, s, H-15); 13C NMR (75 MHz, CDCl3) δ. 181.3 (CO, C-9); 164.3 (C, C-3); 162.4 (C, C-1); 153.7 (C, C-4a); 144.7a(C, C-5); 144.6a (C-10a); 132.0 (C, C-13); 124.0 (CH, C-7); 122.9 (CH, C-12); 121.0 (C, C-8a); 120.1(CH, C-6); 117.0 (CH, C-8); 107.6 (C, C-4); 103.5 (C, C-9a); 94.7 (CH, C-2); 56.3 (OMe, C-16); 25.7(CH3, C-15); 21.9 (CH2, C-11); 18.0 (CH3,C-14). aInterchangeable; LRCIMS (CH4) m./z. (%), 327 [M+] (8), 307 (32), 289 (15), 219 (4), 165 (5), 154 (97), 136 (65), 120 (10), 107 (19), 89 (18); HRCIMS (CH4) m./z. 326.1154 [M + H]+ (calcd. for C19H18O5 326.11542). Copies of the original spectra can be obtained from the corresponding author.
Assays
All three assays were based on inhibition of growth of the parasites by added compound or extract. A DNA-based assay, using PicoGreen® with 485-nm excitation and 528-nm detection, measured the growth of P. falciparum. (Indochina clone W2, chloroquine-resistant) in erythrocytes (Corbett et al., Citation2004). For the T. cruzi. assay, we used a β.-galactosidase–expressing, transgenic T. cruzi. (Tulahuen strain, clone C4). Growth of the intracellular form of T. cruzi. was determined from the cleavage of chlorophenol red-β.-D-galactopyranoside (CPRG, 570 nm) (Torres et al., Citation2003). Growth of the infective form of Leishmania mexicana. (WHO-MOHM/B2/82/BELZ), promastigotes, was assayed based on the reduction of the tetrazolium salt analogue, XTT, and measured at 450 nm (Williams et al., Citation2003).
Results and Discussion
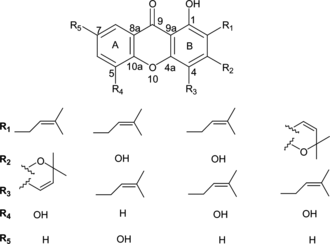
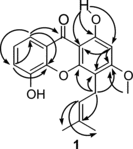
The MeOH liquid-liquid partition fraction of the crude MeOH-EtOAc extract showed significant antiplasmodial activity, and it was selected for bioassay-guided fractionation yielding the novel prenylated xanthone (1) and the four known compounds 2–5 (), whose structures were determined by MS, UV, IR, 1D and 2D NMR data and by comparisons with the literature data (Govindachari et al., Citation1971; Somanathan et al., Citation1974; Linuma et al., Citation1996; Bayma et al., Citation1998). Compound 1 was obtained as a colorless amorphous powder and showed a molecular ion from HRCIMS at m./z. 326.11504 (C19H18O5, calcd. 326.11542), which was consistent with the 19 signals observed in the 13C NMR spectrum. The UV spectrum of compound 1 exhibited four absorption bands, 370, 319, 244, and 211 nm, characteristic of xanthones (Hostettmann et al., Citation1989), while the IR spectrum showed bands at 3510, 3200br, 1650, and 1608 cm−1, suggesting the presence of carbonyl and hydroxyl groups. The 1H NMR spectrum showed four signals in the aromatic region: one singlet, two double doublets, and a triplet revealing a trisubstituted pattern of the xanthone nucleus. A methoxy group was present (δ. 56.3). In addition, one hydroxy group [δ. 5.76 (1H, s)] and a hydrogen bonded hydroxyl group [δ. 12.93 (1H, s, hydrogen bonded OH)] were present. The heteronuclear multiple-bond correlation (HMBC) spectrum () revealed an ABX system of protons of the A ring, which appeared at δ. 7.75, was correlated with the carbonyl carbon (δ. 181.3) to one CH aromatic carbon (δ. 120.1) and to an oxygenated aromatic carbon (δ. 144.7 or 144.6). We conclude that one aromatic proton (δ. 7.75) was located at a peri.-position (C-8) relative to the carbonyl group and that the C-5 position bears an OH substituent. The other aromatic proton, which showed a triplet signal (δ. 7.24), was attributed to H-7 and showed a cross-peak with an oxygenated aromatic carbon (δ. 144.7 or 144.6).
The substitution pattern of the B ring of the xanthone nucleus was confirmed as follows: In the HMBC spectrum, the hydrogen-bonded OH proton (δ. 12.93) showed cross-peaks with two aromatic carbons C-9a and C-2 (δ. 103.5 and 94.7, respectively). The H-2 aromatic proton showed a singlet at δ. 6.40. HMBC correlations were observed between H-2 and C-4 and C-9. The C-3 carbon (δ. 164.3) was correlated with the methoxy protons (δ. 3.95) and methylene protons (δ. 3.50) of the 3,3-dimethylallyl group of the chromene ring. The HMBC spectrum also indicated that the methoxyl group was bound to the C-3 position of the chromene ring. The structure 1 was also supported by the other correlations in the HMBC spectrum. Assignment of the 13C NMR spectral data is shown in the experimental section.
Compunds 3 and 5 showed the highest antiplasmodial activity (IC50 values of 19.7 ± 1.8 and 15.9 ± 3.7 µM, respectively). Levels of activity are similar to those previously reported for other xanthones (Ignatushchenko et al., Citation1997Citation2000). Three of four xanthones tested (compounds 2, 3, and 4) showed activity against T. cruzi., the causative agent of Chagas disease. This is the first report of anti-T. cruzi. activity for these compounds (). None of the compounds in this study showed measurable activity when tested against Leishmania mexicana..
Table 1.. Activity of xanthones 1–5a..
Acknowledgments
Funding for this work comes from the International Cooperative Biodiversity Groups Program (grant no. IUOI TWO1021-01), from the National Institutes of Health, National Science Foundation, and U.S. Department of Agriculture. We also gratefully acknowledge the Smithsonian Tropical Research Institute Equipment Fund for partial funding for the Bruker Avance NMR.
References
- Bayma JC, Arruda M PS, Nieto MS (1998): A prenylated xanthone from the bark of Symphonia globulifera.. Phytochemistry 49: 1159–1160.
- Coley PD, Heller MV, Aizprúa R, Araúz B, Flores N, Correa M, Gupta M, Solis PN, Ortega-Barría E, Romero LI, Gómez B, Ramos M, Cubilla-Ríos L, Capson T, Kursar TA (2003): Using ecological criteria to design plant collection strategies for drug discovery. Front Ecol Environ 1: 421–428.
- Corbett Y, Herrera L, González J, Cubilla-Ríos L, Capson TL, Coley PD, Kursar TA, Romero LI, Ortega-Barría E (2004): A novel DNA-based microfluorimetric method to evaluate anti-malarial drug activity. Am J Trop Med Hyg 70: 119–124.
- Hostettmann K, Hostettmann M (1989): Xanthones. In: Harborne JB, ed., Methods in Plant Biochemistry, Vol. 1. London, Academic Press, pp. 493–508.
- Gelb M, Hol W (2002): Drugs to combat tropical protozoan parasites. Science 297: 343–344.
- Govindachari TR, Kalyanaraman PS, Muthukumaraswany N, Pai BR (1971): Xanthones of Garcinia mangostana.. Tetrahedron 27: 3919–3926.
- Ignatushchenko M, Winter R, Bächinger H, Hinrichs D, Riscoe M (1997): Xanthones as antimalarial agents; studies of a possible mode of action. FEBS Lett 409: 67–73.
- Ignatushchenko M, Winter R, Riscoe M (2000): Xanthones as antimalarial agents: Stage specificity. Am J Trop Med Hyg 62: 77–81.
- Kolodziej H, Kayser O, Kiderlen AF, Ito H, Hatano T, Yoshida T, Foo LY (2001): Antileishemanial activity of hydrolysable tannins and their modulatory effects on nitric oxide and tumour necrosis factor-alpha release in macrophages in vitro.. Planta Med 67: 825–832.
- Linuma M, Tosa H, Tanaka T, Riswan S (1996): Three new xanthones from the bark of Garcinia dioica.. Chem Pharm Bull 44: 232–234.
- Montenegro H, Gutiérrez M, Romero LI, Ortega-Barría E, Capson T, Cubilla-Ríos L (2003): Aporphine alkaloids from Guatteria. spp. with leishmanicidal activity. Planta Med 69: 677–679.
- Ortega-Barría E (2003): Trypanosoma species. In: Long S, Prober C, Pickering L, eds., Principles and Practice of Pediatric Infectious Disease, 2nd ed. London, Churchill Livingstone, pp. 1324–1330.
- Ridley R (2002): Medical need scientific opportunity and the drive for antimalarial drugs. Nature 415: 686–693.
- Somanathan R, Sultanbawa M, Uvais S (1974): Chemical investigation of Ceylonese plants. VIII. Trapezifolixanthone a new diisoprenylated xanthone from the bark of Calophyllum trapezifolium. (Guttiferae). J Chem Soc Perkin Trans 1 Bioog Chem 22: 2515–2517.
- Sultanbawa MU S (1980): Xanthonoids of tropical plants. Tetrahedron 36: 1465–1506.
- Torres D, Ureña L, Ortega-Barría E, Capson T, Cubilla-Ríos L (2003): Five new cassane diterpenes from Myrospermum frutescens. with activity against Trypanosoma cruzi.. J Nat Prod 66: 928–932.
- Winter RW, Cornell KA, Johnson LL (1996): Potentiation of the anti-malarial agent rufigallol. Antimicrob Agents Chemother 40: 1408–1411.
- Williams C, Espinosa OA, Montenegro H, Cubilla-Ríos L, Capson TL, Ortega-Barría E, Romero LI (2003): Hydrosoluble formazan XTT: Its application to natural products drug discovery for Leishmania.. J Microbiol Methods 55: 813–816.
- World Bank (1993): World Development Report.. Investing in Health., New York, Oxford University Press, pp. 329.