Abstract
Eclipta alba. (Linn.) Hassk. (Asteraceae), popularly known as “bhringraj,” is effective against liver damage caused by various hepatotoxins and is used traditionally in Indian phytoformulations. For enhanced in vitro. production of wedelolactone (W), leaves of aseptically germinated seedlings were cultured on Murashige and Skoog's (MS) medium supplemented with plant growth regulators under controlled and standardized chemical and environmental conditions. The wedelolactone was extracted, isolated, purified, chemically characterized, and quantitatively estimated. Rats were divided into four groups of six animals each and given paracetamol (250 mg/kg, p.o.), paracetamol (250 mg/kg, p.o.) with wedelolactone (100 mg/kg, p.o.), paracetamol (250 mg/kg, p.o.) with plant leaves extract (250 mg/kg, p.o.), paracetamol (250 mg/kg, p.o.) with leaf callus extract (250 mg/kg, p.o.), respectively. Blood samples were collected at 1, 2, 3, 4, and 6 h after drug administration, and plasma paracetamol concentration was determined. Pharmacokinetic studies revealed that concomitant administration of wedelolactone with paracetamol does not affect the bioavailability of paracetamol. There was no change in Cmax between paracetamol and paracetamol with wedelolactone, but the Tmax of paracetamol was 2 h in control animals while administration of wedelolactone and extracts with paracetamol resulted in shifting of Tmax from 2 to 3 h without affecting the area under the curve (AUC). Because wedelolactone does not alter the bioavailability of paracetamol significantly and also shows a hepatoprotective effect, its use may be recommended in prolonged paracetamol therapy or paracetamol toxicity.
Introduction
Eclipta. is a small genus of herbs distributed in the tropical and subtropical regions. Eclipta alba. (Linn.) Hassk. (syn., Eclipta prostrata. L.) (Asteraceae) is a small branched annual herb with white flower heads that is found in moist areas throughout India ascending up to 600 feet on the hills. Commonly, it is known as “white bhringraja” when in flower, and as “black bhringraja” when in fruit (Bhargava & Seshadri, Citation1972). Herb of Eclipta alba., traditionally used against jaundice (Chopra et al., Citation1966), contains coumestans derivatives, that is, wedelolactone (W) and demethylwedelolactone (DMW), possessing potent antihepatotoxic activity (Wagner et al., Citation1986; Saxena et al., Citation1993). The herb also clears the tubules of kidney and ureters (Karnick, Citation1989), possesses myocardial depressant, antihypertensive effects (Gupta et al., Citation1976), and has shown anti-inflammatory activity in rats (Lahkar, Citation1981). Fixed oil of Eclipta alba. cures vitilgo (leucoderma) and insomnia (Kulkarni, Citation1990; Karnick & Kulkarni, Citation1990).
Eclipta alba. is a seasonal plant that is difficult to cultivate. At present, the availability of these coumestans is becoming increasingly limited. Therefore, it was thought worthwhile to develop callus and other cultures. The current work was undertaken with the following objectives:
To investigate the pharmacokinetic interaction of wedelolactone with paracetamol.
To minimize the toxic effect of paracetamol by concomitant administration of wedelolactone with paracetamol without affecting paracetamol's bioavailability during the routine therapy or in paracetamol-induced hepatotoxicity.
To check the presence of other molecule(s) in the extracts that can enhance or suppress the antihepatotoxic effects of wedelolactone.
Materials and Methods
Seed viability and vigor studies
Viable seeds were collected from mature plants grown in moist areas of the Maharshi Dayanand University campus in the months of October–November. The plants were identified by the Department of Biosciences, Maharshi Dayanand University (Rohtak, Haryana, India). The voucher specimens of seeds and plant were deposited in the herbarium section of the Pharmacognosy Department, Faculty of Pharmaceutical Sciences, Maharshi Dayanand University.
The following methods were used to assess the viability and vigor:
(a) Triphenyltetrazolium chloride staining method (TTC or tetrazolium salt) (Dixon, 1994). Seeds were soaked in distilled water for 2 days and were split longitudinally into equal halves with a scalpel. One half of each seed was kept in 1% aqueous solution of 2,3,5-triphenyl-tetrazolium chloride for 5–6 h. Seeds were then washed with distilled water and the number of half seeds stained red was recorded for calculation of the viability percentage as follows:
(b) By germination without surface sterilization. Seeds were washed under running tap water. The seeds were then dipped into double-distilled water (unsterilized). Washed seeds were transferred into Petri dishes containing absorbent cotton pad with a filter paper over it. Gibberellic acid solution (1% or 2%) was poured, covered with aluminum foil, and kept for germination in a seed germinator (Hicon Instruments, Grover Enterprises, New Delhi, India) under dark at 25 ± 2°C. Both seed viability and vigor were calculated by following formulae:
where a., b., c., and d., respectively, represent the number of seeds that germinated after 1, 2, 3, and 4 days, and S. represents the total number of seeds to be germinated.
Aseptic seed germination
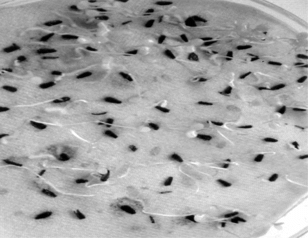
The viable seeds of Eclipta alba. were treated with very dilute detergent solution (Cedepol). They were washed under running tap water followed by sterile double-distilled water treatment and then sterilized with silver nitrate (0.5%) or sodium hypochlorite (1%) and germinated aseptically in dark at 25 ± 2°C in a seed germinator (Hicon Instruments) ().
Initiation and development of leaf callus
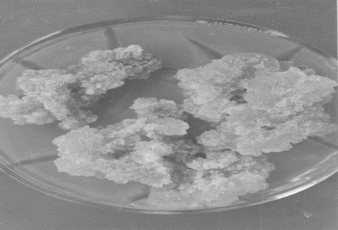
Callus cultures of Eclipta alba. were established from excised leaf explants of aseptically germinated seedlings and grown in sterile conditions on Murashige and Skoog's (Citation1962) medium. Medium supplemented with 2,4-dichlorophenoxyacetic acid (Loba Chemicals, Bombay, India) and 6-benzyladenine (Loba Chemicals) were found very effective for both initiation and development of callus (; ).
Table 1. Effects of plant growth regulators on initiation of callus in MS medium.
Figure 2 Initiation of callus from leaf explant of germinated seedling after 15 days in MS medium supplemented with 2,4-D (4.52 mg/L) + 6-BA (4.43 mg/L).

Medium supplemented with 2,4-D (13.58 mg/L) and 6-BA (4.43 mg/L) along with ascorbic acid (1 g/L) gave the best combination for development of callus cultures (). These cultures were incubated under controlled conditions: temperature 25 ± 2°C; light regime 16/8 h; light intensity 1600 lux; humidity 60–65%. For maintenance of callus cultures, developed calli were maintained on plant growth regulator free medium ().
Table 2. Effects of plant growth regulators on development of callus in MS medium.
Identification and assay of coumestans
The lyophilized leaf callus and dried aerial parts of intact plant (4 months old) were Soxhlet extracted with MeOH for 36 h. The solvent was removed and the residues were suspended in water separately and heated on a steam bath below 80°C for 30 min. After filtration, the aqueous phase was partitioned with ethyl acetate. The organic phase of both callus and plant material were dried, filtered, and the solvents were evaporated to yield light-brown powder (developing with alcoholic ferric chloride solution a deep-green coloration that turned yellow by addition of concentrated nitric acid on keeping for 15 min) that were dried over anhydrous sodium sulfate. The fraction eluted with chloroform:methanol (CHCI3:MeOH, 70:30) was found to contain wedelolactone and demethylwedelolactone, which were separated by preparative TLC (PTLC) (toluene:acetone:formic acid, 11:6:1).
UV spectrophotometric analysis
Purified wedelolactone and demethylwedelolactone (coumestans) were dissolved in HPLC grade methanol (10 ml) and analyzed spectrophotometrically at different wavelengths (near and far UV) using a double-beam spectrophotometer (model 2101; Systronics India Limited, Ahmadabad, Gujarat, India).
Qualitative and quantitative HPLC analysis of wedelolactone
For HPLC analysis of wedelolactone, a Waters HPLC system was used with photodiode-array detector: Column: Hibar LiChlorspher 100 C-18, 125 × 4 mm ID, stainless steel: mobile phase comprised A, water (freshly distilled, HPLC grade) and B, acetonitrile (Chrom-AR quality). A and B were mixed with 10 ml 0.1 N phosphoric acid/L; from 15% B to 40% B in 25-min linear gradient. Flow was adjusted to 1 ml/min with injection volume: 10 µl in MeOH.
For calibration curves, 1, 2, 4, 6, 8, and 10 μl of pure wedelolactone were injected and peak height and peak area were recorded.
Induction of hepatotoxicity in albino rats
Animals
Wistar albino rats (200–250 g) of either sex were maintained under standard environmental conditions (23 ± 2°C, 55 ± 10% relative humidity, 12-h light/dark cycle). They were allowed standard laboratory feed and water ad libitum.. Groups of six rats each were used in all sets of experiments.
Paracetamol induced hepatotoxicity and treatment schedule
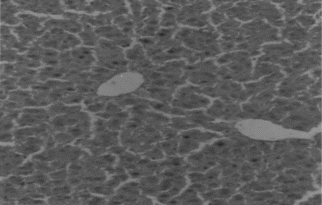
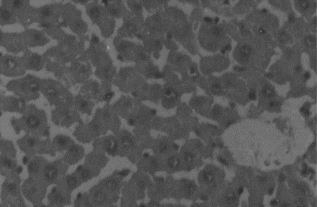
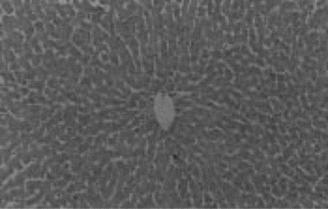
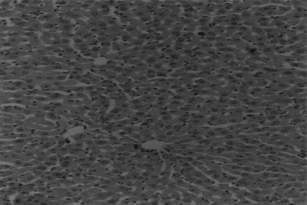
Animals in group I were given normal saline/vehicle control (1 ml/kg, p.o.). On the eighth day they were sacrificed, and biochemical parameters were estimated. Liver sections were taken out for histopathological studies ().
Animals of groups II to VII served as a model for paracetamol (PCM)-induced hepatotoxicity. These animals were dosed with paracetamol (500 mg/kg, p.o.) (daily dose for 1 week). In addition to this, the following treatments were given to animals of groups III to VI for a 1-week period:
Group II: PCM control (500 mg/kg body weight, p.o., daily dose for 1 week)
Group III: Plant leaves extract (250 mg/kg body weight, p.o.)
Group IV: Leaf callus extract (250 mg/kg body weight, p.o.) ()
Group V: Wedelolactone: (100 mg/kg body weight, p.o.) ()
Group VI: Demethylwedelolactone: (100 mg/kg body weight, p.o.)
Group VII: Standard herbal formulation: silymarin (100 mg/kg body weight, p.o.) ()
Assessment of liver functions
All the animals were anesthetized with ether, and blood was withdrawn from retro-orbital sinus after 1 week of paracetamol treatment and 1-week treatment with the ethanol extracts and drugs. The blood samples were centrifuged at 3000 rpm at 4°C for 10 min to separate the serum. Biochemical parameters, that is, serum glutamyl oxaloacetate transaminase (SGOT), serum glutamyl pyruvate transaminase (SGPT), alkaline phosphatase (AKLP), serum albumin, total protein (T. Prot), and total bilirubin (T. Bil), were analyzed according to reported methods. Histopathological studies were also carried out.
Pharmacokinetic interaction studies
Methods available for estimation of paracetamol in blood plasma
The following methods are available for the estimation of plasma paracetamol:
UV spectrophotometric methods (based on extinction due to the compound itself).
Based on the formation of a colored reaction product or diminution of color.
Gas-liquid chromatography method.
High-performance liquid chromatographic (HPLC) method.
Brodie's UV spectroscopic method for estimation of paracetamol in blood plasma
This method includes some form of extraction procedure before quantitation by measuring extinction produced due to the drug. Methanol is the solvent used for this purpose, and extraction is aided by prior saturation of the plasma with a salt, usually sodium chloride. Brodie extracted paracetamol directly into methanol, which precipitates proteins of plasma. After extraction back into sodium hydroxide, the solution was acidified and heated to achieve hydrolysis. The resulting p.-aminophenol is diazotized and coupled to α.-naphthol, giving red-violet coloration that could be measured at 510 nm.
In this method, 1.0 ml of plasma is taken in a 15-ml centrifuge tube containing 1.0 ml of water. Solid 1 g sodium chloride (NaCI) and 10 ml of methanol was added. The tube was closed and shaken for 15 min on a mechanical shaker. The tube was centrifuged for 5 min at 2000 rpm. An 8-ml aliquot of the methanol layer was transferred to a 12-ml centrifuge containing 1.0 ml of 0.1 N sodium hydroxide (NaOH). Tube was shaken for 5 min and then centrifuged for 5 min. Methanol was removed and an aliquot of 0.8 ml of the NaOH layer was transferred to a 12-ml centrifuge tube containing 0.2 ml conc. hydrochloric acid (HCl). The tube was covered, heated at 100°C for 30 min, and cooled. To the cooled tube, 0.5 ml of 0.2% sodium nitrate solution was added, mixed gently, allowed to stand for 20 min, and then 0.5 ml of 1.0% ammonium sulfamate solution was added. Contents were mixed and allowed to stand for 3 min. Finally, 0.1 ml of 12% α-naphthol in ethyl alcohol and 2.0 ml of 5.5 N NaOH were added. The contents were mixed and tube cooled in cold water for 5 min. The absorbance was then recorded at a wavelength of 510 nm.
Preparation of standard plot for paracetamol by UV spectrophotometric analysis
For a standard plot, different samples of paracetamol (0.0, 1.0, 2.0, 4.0, 6.0, 8.0, 10.0, 12.0, 14.0 µg/ml) were prepared in HPLC grade distilled water and analyzed UV spectroscopically at 510 nm ().
Table 3. Standard plot data for estimation of paracetamol by spectrophotometric method.
Statistical analysis
The data represented in are mean ± SE (n = 6). The difference between the drug- treated groups and toxic control group was evaluated by Student's t.-test. p < 0.05 was regarded as significant.
Table 4. Antihepatotoxic effects of ethanol extract of plant leaves, leaf callus, wedelolactone, demethylwedelolactone, and silymarin on biochemical parameters in paracetamol-induced hepatic injury.
Results and Discussion
Seed viability and vigor were found to be 100% as all the seeds germinated within 24 h (). Auxins, that is, 2,4-d-chlorophenoxyacetic acid (2,4-D), was found very effective in the initiation and development of callus, and the effect was increased with the addition of low concentration of cytokinin, that is, 6-benzyladenine (6-BA). The best callus initiation in all types of explants was obtained in MS medium supplemented with 2,4-D and 6-BA at concentrations of 4.52 and 4.43 mg/L, respectively (; ). It was found that auxins or combination auxin/cytokinin supported the callus development. Medium supplemented with 2, 4-D (13.58 mg/L) and 6-BA (4.43 mg/L) along with ascorbic acid (1 g/L) gave the best combination for development of callus cultures (; ).
The following qualitative tests confirmed the presence of these coumestans:
Wedelolactone and demethylwedelolactone produced deep-green coloration with alcoholic ferric chloride solution. This green color turned yellow on further addition of concentrated nitric acid (on keeping for 15 min).
Wedelolactone and demethylwedelolactone were soluble in sodium bicarbonate solution and produced a brown color.
In spectrotometric analysis, the UV λmax was found to be 351, 248, and 208 nm for wedelolactone, and 351, 249 and 208 for demethylwedelolactone. Observed physical and chemical properties of wedelolactone and demethylwedelolactone are as follows. Wedelolactone: m.p., 310°C (uncorrected); TLC (Rf), 0.58 (dichloromethane: methanol: water, 14:9:4); 0.63 (toluene: acetone: formic acid, 11:6:1) bright-blue fluorescence; 5% ferric chloride solution, green; UV max (nm) (MeOH), 209, 249, 302, 351: (NaOAC): 378, decreased intensity; (NaOAc/H3B“O”3): 360 (O-di-OH): (NaOMe): 378, decreased intensity; IR (KBr) V(cm−1) : 3300, 1710 (δ-lactone-carbonyl), 1635, 1610, 1445, 1420, 1320, 1200, 1150, 1070; MS (4 KV, 70 eV, 50 µ: EI, 200°C) m./e. (rel. int. %): 314 (M+) (100), 313 (10), 299 (M+–CH3) (22), 285 (12), 271 (M+–CH30) (8), 243 (M+–CH3–CO–CO) (31), 187 (17), 69 (42).
Demethywedelolactone: TLC (Rf): 0.47 (dichloromethane:methanol:water, 14:9:4); 0.50 (toluene:acetone:formic acid, 11:6:1) white-blue fluorescence, ferric chloride solution: green; UV λmax (nm) (MeOH): 209, 249, 302sh, 351: (NaOAC): 372, decreased intensity; (NaOAc/H3B“O”3): 360 (O-di-OH): (NaOMe): 372, decreased intensity; IR (KBr) V(cm−1): 3300–3200 (OH), 1710 (δ-lactone-carbonyl), 1620 (d), 1480, 1450, 1335, 1280, 1150, 1080, 890; MS: m./e. (rel. int. %): 300 (M+) (100), no significant fragmentation. The 1H NMR spectrum of the acetate showed four acetoxy groups and two pairs of aromatic protons at 7.03/7.25 and 7.55/7.95, two meta. and two para. related, corresponding with protons at positions 6, 8, 10, and 13 of the coumestan nucleus.
In HPLC analysis, the observed retention times for wedelolactone and demethylwedelolactone were 11 and 17.5 min, respectively.
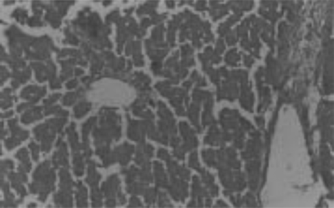
Hepatotoxicity due to paracetamol toxicity was detected 24 h after ingestion, and there was a good correlation between liver damage and plasma paracetamol concentration 4 h and 12 h after ingestion. An equally valid indication was obtained from the half-life of paracetamol in plasma. Acute overdosage of paracetamol caused potentially fatal necrosis (). Plasma amino-transferases were elevated, and the concentration of billirubin in plasma was increased. Biopsy of the liver revealed centilobular necrosis with sparing of the peritoneal area. Paracetamol damaged the liver through the formation of highly reactive metabolite that normally is inactivated by conjugation with glutathione. On overdosage of paracetamol or in paracetamol- induced hepatotoxicity, glutathione stores are exhausted, allowing the accumulation of toxic metabolites that covalently bind with cellular elements and cause necrosis of hepatocytes. Liver necrosis was localized to centrilobular areas, and in some animals confluent necrosis developed.
The hepatoprotective effects of Eclipta alba. plant leaf extract, leaf callus extract, wedelolactone, demethylwedelolactone, and standard reference drug, that is, silymarin, against paracetamol-induced hepatotoxicity in rats are recorded in . Wedelolactone showed comparatively better antihepatotoxic effects than other drug samples (). It cured the animals by alleviating the biochemical parameters and by recovering normal anatomical architecture. It possesses a significant membrane-stabilizing property, antioxidant property, and also enhances protein synthesis in liver cells.
Systemic bioavailability of paracetamol after oral administration was incomplete due to first-pass metabolism. The proportion of a dose reaching the systemic circulation as unchanged paracetamol appears to be depend on the amount given, decreasing from about 90% after 1000 to 2000 mg paracetamol to about 70% after 500 mg. After 8 h, only small amounts of unchanged paracetamol were detectable in plasma. The half-life in plasma was about 2 h after different doses.
Pharmacokinetic studies revealed that administration of wedelolactone with paracetamol in plasma did not change the bioavailability of paracetamol. There was no change in Cmax between paracetamol and paracetamol with wedelolactone, but the Tmax of paracetamol was 2 h in control animals while administration of wedelolactone with paracetamol resulted in shifting of Tmax to 2–3 h without significantly affecting the area under the curve (AUC) ().
Table 5. Peak plasma concentration (Cmax), time of peak concentration (Tmax), and area under the curve (AUC) of paracetamol.
Finally, it has been concluded that wedelolactone possesses a significant/potent antihepatotoxic property, even better than a reference standard (i.e., silymarin). The pharmacological mechanisms of both paracetamol and wedelolactone are different. Moreover, as wedelolactone does not alter the bioavailability of paracetamol significantly, and has hepatoprotective effects, its use may be recommended in prolonged paracetamol therapy or paracetamol toxicities.
Acknowledgments
We would like to extend our sincere thanks to the Department of Biosciences, Maharshi Dayanand University, Rohtak, Haryana, for pursuing authentication of plant material. Financial assistance from All India Council for Technical Education (AICTE), New Delhi, is gratefully acknowledged.
References
- Bhargava KK, Seshadri TR (1972): Wedelolactone and demethylwedelolactone from Eclipta alba.. J Res Indian Med 9: 9–12.
- Chopra RN, Nayar SL, Chopra IC (1966): Glossary of Indian Medicinal Plants, New Delhi, India, Council of Scientific and Industrial Research, pp. 104–105.
- Das M, Bhavsar GC, Chawhan MG (1990): Spectrophotometric determination of hepatoprotective principles of Eclipta alba.. Indian Drugs 28: 100–102.
- Dixon RA (1984): Callus and suspension culture. In: Dixon RA, ed., Plant Cell Culture: A Practical Approach, ( Practical Approach Series). Oxford, Washington, DC, IRL Press, pp. 14–20.
- Gupta SC, Bajaj UK, Sharma VN (1976): Cardiovascular effects of Eclipta alba. (Hassk.) Bhringraja. J Res Indian Med Yoga Homeopathy 13: 91–95.
- Karnick CR (1989): On comparative values of Indian and Chinese medicinal plants. J Natl Integrated Med Assoc 31: 13–16.
- Karnick CR, Kulkarni M (1990): Ethnobotanical studies of some medicinal plants used in skin diseases. Maharastra Med J 37: 131–134.
- Kulkarni PH (1990): Ayurvedic therapy: Insomnia. Deerghayu International 6: 21.
- Lahkar S (1981): Clinical trials of Rumalaya in osteoarthritis of knee. J Med Surg 21: 21–22.
- Murashige T, Skoog F (1962): A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15: 473–497.
- Saxena AK, Singh B, Anand KK (1993): Hepatoprotective effects of Eclipta alba. on subcellular levels in rats. J Ethanopharmacology 40: 155–161.
- Wagner H, Geyer B, Kiso Y, Rao GS (1986): Coumestans as the main active principle of the liver drug Eclipta alba. and Wadelia calendulacia.. Planta Med 52: 370–373.