Abstract
We investigated the antihypertensive effect of a methanol extract (ME) of fenugreek seeds and its methanol fraction (MF) in deoxycorticosterone acetate (DOCA)-salt-induced and fructose-induced hypertensive rats. In the DOCA model, DOCA (15 mg/kg, twice a week; s.c.) was administered in uni-nephrectomized animals for 4 weeks. Methanol extract of fenugreek seeds (30 mg kg−1 day−1; p.o.) and its methanol fraction (15 mg kg−1 day−1; p.o.) were evaluated for their antihypertensive effect. In the fructose model, drinking water was replaced with a 10% fructose solution for 6 weeks to induce hypertension. ME (100 mg kg−1 day−1; p.o.) was assessed for its antihypertensive effect in the fructose model. After completion of the treatment schedule, blood pressure and vascular reactivity to various agonists like serotonin (5-hydroxytryptamine; 5-HT), noradrenaline (NA), and adrenaline (Adr) were recorded in rats of both models. A dose-response curve (DRC) of 5-HT was carried out in isolated rat fundus strip of the fructose-induced hypertensive rats. Chronic administration of ME (30 mg kg−1 day−1; p.o.) and MF (15 mg kg−1 day−1; p.o.) of fenugreek seeds significantly reduced blood pressure in DOCA salt and ME (100 mg kg−1 day−1; p.o.) reduced blood pressure in fructose-induced hypertensive rats. Treatment with ME (100 mg kg−1 day−1; p.o.) in fructose model for 6 weeks shifted the DRC toward the right on rat fundus. Thus, fenugreek seeds exhibit a significant antihypertensive effect. The mechanism of action may partly involve the serotonergic antagonistic property involving the 5-HT2 receptor subtype.
Introduction
Trigonella foenum-graecum. L. (Leguminosae), commonly known as fenugreek, is native to the area from the eastern Mediterranean to Central Asia and Ethiopia and is cultivated in Pakistan, India, and China (Morton, Citation1990). The seeds are hot with a sharp, bitter taste. They are used for their carminative, tonic, laxative, and expectorant properties. They possess antipyretic, anthelmintic, appetite stimulant (Kirtikar & Basu, Citation1993), aphrodisiac (Chopra et al., Citation1982), antifatigue (Jha, Citation2003), hypocholesterolemic, and hypoglycemic activities (Ribes, Citation1984; Sharma, Citation1986). Fenugreek seeds and leaves are also said to have antidiabetic (Shani et al., Citation1974), antiulcer (Al Meshal et al., Citation1985), and antihypertensive properties (Ghosal et al., Citation1974).
The seeds of Trigonella foenum-graecum. contain the alkaloid trigonelline with mucilage, tannic acid, yellow coloring matter, fixed and volatile oils, and a bitter extractive diosgenin, gitogenin, a trace of trigogenin and vitamin A (Jayaweera, Citation1981). Selye and Bois were the first to demonstrate that deoxycorticosterone acetate (DOCA) produces hypertension in rats (Selye & Bois, Citation1957). There is increased DOCA-induced reabsorbtion of salt and water leading to increased blood volume and hence increased blood pressure (BP). It has been hypothesized that 5-HT2B receptor is upregulated and necessary for maintaining elevated blood pressure in rats made hypertensive by DOCA and N.-nitro-L-arginine methyl ester (NAME; nitric oxide synthase inhibitor) (Banes & Watts, Citation2003). Physiologically, this upregulation is important because 5-HT has a 300-fold greater affinity for 5-HT2B receptor compared with 5-HT2A receptor (Wainscott et al., Citation1993).
Fructose is widely present in numerous foods. It has been commonly used as a sweetener and promoted as being useful for weight reduction, exercise endurance, and diabetes (Dai & McNeill, Citation1995). It has been demonstrated that hypertension develops when normal rats are fed with a fructose-enriched diet (Verma et al., Citation1994; Suzuki et al., Citation1997).
Fenugreek has not been highly explored for its therapeutic profile in hypertension. Therefore, the objective of our current study was to investigate its antihypertensive activity using DOCA-salt-induced fructose-induced hypertensive models in rats.
Materials and Methods
Preparation of extract
Fenugreek seeds (1 kg) were purchased locally and authenticated. Seeds were crushed to a coarse powder and defatted with petroleum ether (60–80°C) using a Soxhlet extractor. The marc obtained was extracted with methanol. The methanol extract (5.85% yield) was separated into acetone-soluble (0.95% yield) and acetone-insoluble (4.90% yield) fractions. The acetone-soluble fraction was charged into a chromatography column of neutral alumina. The column was eluted with ethyl acetate (0.002% yield), methanol (0.06% yield), and dimethylsulfoxide (DMSO) (0.1% yield). The methanol extract (ME) and its methanol fraction (MF) were used for the study. A voucher specimen has been deposited at Botonical Survey of India, Pune, for future reference. Phytochemical tests of ME and MF indicated the presence of alkaloids and saponins.
Animals
Wistar rats of either sex (200–250 g) were housed in plastic cages in groups of six under standard lab conditions of temp 25±1°C with 12 h light/dark cycle. Animals had free access to standard chow diet and water ad libitum.. They were then trained for a week to become accustomed to the procedure of indirect blood pressure measurement. The institutional animal ethical committee approved the protocol of the study.
Drugs
Noradrenline, adrenaline, 5-HT, urethane, and DOCA were purchased from Sigma (Sigma Chemicals, St. Louis, MO, USA). Fructose was purchased from SD Fine Chemicals (Mumbai, India). DOCA was dispersed in cottonseed oil. Dilutions of ME and MF of fenugreek seeds were prepared in fresh distilled water and administered orally. All drug solutions were freshly prepared in distilled water before each experiment. Fructose solution (10%) was freshly prepared in tap water.
Induction of hypertension
DOCA-salt-induced hypertension
Hypertension was induced experimentally in female Wistar rats (200–250 g) by unilateral nephrectomy (Nagawa & Nasjletti, Citation1988). Rats were anesthetized with ether, and a lateral incision was made in the area overlying the kidney. The renal blood vessel was ligated with fine sterile silk thread and the kidney was removed. The incision was sutured and closed with Michel clips. All operated rats received an injection of ampicillin (10 mg/kg, i.p.) daily for 5 days. Neosporin powder (polymyxin B sulfate BP, zinc bacitracin BP, neomycin sulfate IP) was applied locally to prevent infection. One week later, DOCA (15 mg/kg, twice a week; s.c., for 4 weeks) dispersed in cottonseed oil was injected to uni-nephrectomized rats. A solution of 1% saline + 0.2% KCl was given ad libitum. was given instead of drinking water. In sham-operated control animals, a similar procedure was performed except the treatment with DOCA.
Fructose-induced hypertension
Hypertension was induced experimentally in male Wistar rats (200–250 g) by high-fructose diet or by giving 10% fructose solution to drink ad libitum. for 6 weeks. Fructose solution was prepared every 2 days by dissolving the fructose in tap water. Ordinary tap water was given to control animals to drink throughout the whole experimental period (Vogel, Citation2002).
Experimental protocol
DOCA-salt-induced hypertension model
A total of 36 unilateral nephrectomized female animals were randomized into six groups of six animals each.
Group 1.: Sham control: Sham-operated control rats received 0.2 mL of cottonseed oil, s.c., twice a week for 4 weeks. Drinking water was replaced with 1% saline + 0.2% KCl ad libitum..
Group 2.: DOCA: Unilateral nephrectomized rats received DOCA-salt (15 mg/kg, twice a week, s.c.) dispersed in cottonseed oil for 4 weeks. Drinking water was replaced with 1% saline + 0.2% KCl ad libitum..
Group 3.: DOCA + ME-30: Unilateral nephrectomized rats received DOCA-salt (15 mg/kg, twice a week, s.c.) dispersed in cottonseed oil and ME (30 mg kg−1 day−1, p.o.) for 4 weeks. Drinking water was replaced with 1% saline + 0.2% KCl ad libitum..
Group 4.: ME-30: Unilateral neprectomized rats received ME (30 mg kg−1 day−1, p.o.) and cottonseed oil (0.2 mL/rat, twice a week, s.c.) for 4 weeks. Drinking water was replaced with 1% saline + 0.2% KCl ad libitum..
Group 5.: DOCA + MF-15: Unilateral nephrectomized rats received DOCA-salt (15 mg/kg, twice a week, s.c.) and MF (15 mg kg−1 day−1, p.o.) for 4 weeks. Drinking water was replaced with 1% saline + 0.2% KCl ad libitum..
Group 6.: MF-15: Unilateral nephrectomized rats received MF (15 mg kg−1 day−1, p.o.) and cottonseed oil (0.2 mL/rat, twice a week, s.c.) for 4 weeks. Drinking water was replaced with 1% saline + 0.2% KCl ad libitum..
Fructose-induced hypertension model
A total of 24 male Wistar rats (250–300 g) were randomized and divided into four groups of six each.
Group 1.: Control: Animals received no medication but were given tap water for drinking.
Group 2.: F-10: Animals received 10% fructose solution instead of drinking water ad libitum. for 6 weeks.
Group 3.: F-10 + ME-100: Animals received 10% fructose solution instead of drinking water, ad libitum., with ME (100 mg kg−1 day−1, p.o.) for 6 weeks.
Group 4.: ME-100: Animals received ME (100 mg kg−1 day−1, p.o.) and tap water for drinking for 6 weeks.
Measurement of blood pressure
Measurement of blood pressure by noninvasive (indirect) method
For arterial blood pressure measurement using the tail-cuff method, rats were trained for at least 1 week until the blood pressure was steadily recorded with minimal stress and restraint. The first cardiovascular parameters were discarded and the mean of five or six subsequent measurements were recorded.
Cardiovascular parameters (systolic, diastolic, mean blood pressure, and heart rate) were measured weekly for 5 weeks by indirect noninvasive tail-cuff method using Letica 5002 Storage Pressure Meter (Panlab Blood Pressure Recorder model LE 2002 N, Barcelona, Spain).
Measurement of blood pressure by invasive (direct) method
After 4 weeks of completion of treatment schedule, rats from each group were anesthetized with urethane (120 mg/100 g). The femoral vein was cannulated with a fine polyethylene catheter for administration of drug. Tracheostomy was performed, and blood pressure was recorded from the left common carotid artery using a pressure transducer by the direct method on a BIOPAC Data Acquisition System (BIOPAC MP30 SYSTEM, Santa Barbara, CA, USA). Heparinized saline (250 IU/mL) was filled in the transducer and in the fine polyethylene catheter cannulated to the carotid artery to prevent clotting. After 30 min of stabilization, blood pressure and vascular reactivity to noradrenaline (1 and 2 µM/kg), adrenaline (1 and 2 µM/kg), and 5-HT (1 and 2 µM/kg) were recorded.
In vitro studies.
After completion of treatment schedule in the fructose-induced hypertension model, rats from each group were sacrificed by stunning, and the fundus was removed and placed in Krebs solution. A strip of fundus was mounted in a bath containing Krebs solution. The physiologic salt solution had the following composition (mM): NaCl, 118; KCl, 4.7; CaCl2,2.5; MgSO4,1.2; NaHCO3,25; KH2PO4,1.2; and glucose, 11. The physiologic salt solution had a pH of 7.4. It was warmed to 37°C and aerated with 95% O2 and 5% CO2(Carbogen). One end was tied to an aerator tube and the other end to a forced displacement transducer (Ugo Basile, Comerio, Italy). Each strip was placed under optimum resting tension (1.5 g) and allowed to equilibrate for 30 min with frequent changes of Krebs solution at 10-min intervals. Contractile response to each dose of 5-HT was recorded for 90 s for each tissue preparation on a Gemini Two Channel Recorder 7070 (Ugo Basile) (Goyal, Citation1999).
Statistics
The mean±SEM values were calculated for each group. One-way ANOVA followed by Tukey multiple comparison test was used for statistical analysis. Values of p < 0.05 were considered statistically significant.
Results
Antihypertensive effect of ME-30 and MF-15 on DOCA-salt hypertensive rats
Measurement of blood pressure by noninvasive (indirect) method
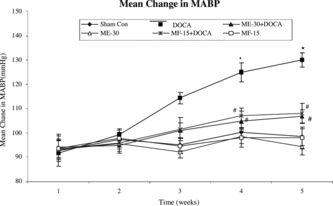
Administration of ME-30 and MF-15 in DOCA-salt unilateral nephrectomized rats significantly (p < 0.05) reduced the mean arterial blood pressure at the end of the third week and showed further reduction at the end of the fourth week as compared with DOCA-salt hypertensive rats alone, implying an antihypertensive effect of ME and MF of fenugreek seeds ().
Figure 1 Time course (weekly) of changes in mean arterial pressure (mm Hg) during 5 weeks in sham control, DOCA, ME-30 + DOCA, ME-30, MF-15 + DOCA, and MF-15 treated groups. *p < 0.05 when compared with sham control group. #p < 0.05 when compared with DOCA hypertensive rats. Vertical lines represent SEM, n = 6.
Measurement of blood pressure by invasive (direct) method
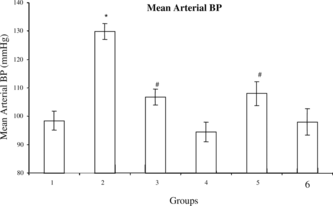
Administration of DOCA for 4 weeks in uni-nephrectomized rats produced a significant elevation (p < 0.05) of MABP (130.0±2.9 mm Hg) as compared with sham control rats (98.4±3.4 mm Hg). In sham-operated control rats, chronic administration of ME-30 and MF-15 for 4 weeks did not alter any BP parameters as compared with sham-operated control rats. However, chronic administration of ME-30 and MF-15 for 4 weeks in uni-nephrectomized DOCA-treated rats significantly (p < 0.05) reduced MABP (ME-30: 106.7±2.8 mm Hg; MF-15: 108.0±4.2 mm Hg) as compared with mean arterial blood pressure (MABP) (130.0±2.9 mm Hg) of DOCA-salt hypertensive rats implying an antihypertensive effect ().
Figure 2 Mean arterial blood pressure (mm Hg) after completion of treatment schedule in (1) sham control, (2) DOCA, (3) ME-30 + DOCA, (4) ME-30, (5) MF-15 + DOCA, and (6) MF-15 treated groups. *p < 0.05 when compared with sham control group. #p < 0.05 when compared with DOCA hypertensive rats. Vertical lines represent SEM, n = 6.
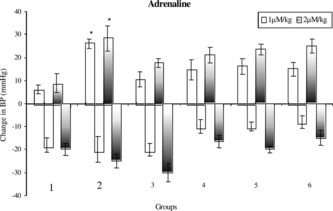
Effect of ME-30 and MF-15 on vascular reactivity to adrenaline, noradrenaline, and 5-hydroxytryptamine in DOCA-salt hypertensive rats
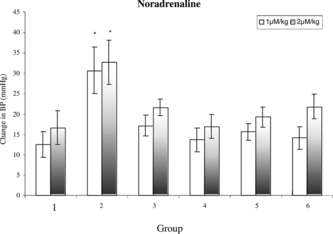
Pressor responses to NA, Adr, and 5-HT were not altered significantly in case of ME-30- and MF-15-treated rats as compared with sham control rats. Pressor responses to NA, Adr, and 5-HT were significantly (p < 0.05) increased in case of uni-nephrectomized DOCA-salt-treated hypertensive rats as compared with sham control rats. Pressor responses to 5-HT was significantly (p < 0.05) reduced in case of uni-nephrectomized DOCA-salt-treated rats that received ME-30 and MF-15 for 4 weeks as compared with DOCA-salt hypertensive rats (.
Figure 3 Mean change in blood pressure to noradrenaline in (1) sham control, (2) DOCA, (3) ME-30 + DOCA, (4) ME-30, (5) MF-15 + DOCA, and (6) MF-15 treated groups. *p < 0.05 when compared with sham control rats. Vertical lines represent SEM, n = 6.
Antihypertensive effect of ME-100 in fructose-induced hypertensive rats
Measurement of blood pressure by noninvasive (indirect) method
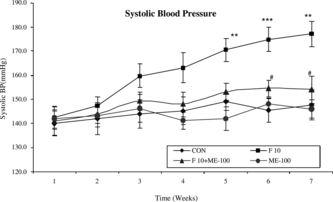
Fructose (10% solution) feeding for 6 weeks in rats produced a significant (p < 0.01) elevation of systolic blood pressure (SBP) (177±5.3 mm Hg) as compared with control rats (147.6±6.2 mm Hg). In control rats, chronic administration of ME-100 for 6 weeks did not alter SBP (146±3.8 mm Hg) as compared with control rats (147.6±6.2 mm Hg). However, chronic administration of ME-100 for 6 weeks in fructose-fed rats significantly (p < 0.05) reduced SBP (154.1±5.7 mm Hg) as compared with fructose-fed hypertensive rats (177±5.3 mm Hg) implying an antihypertensive effect ().
Figure 6 Time course (weekly) of changes in systolic blood pressure (mm Hg) during 7 weeks in control (CoN), F-10, F-10 + ME-100, and ME-100 treated groups. **p < 0.01, ***p < 0.001 when compared with control rats; #p < 0.05 when compared with fructose hypertensive rats. Vertical lines represent SEM, n = 6.
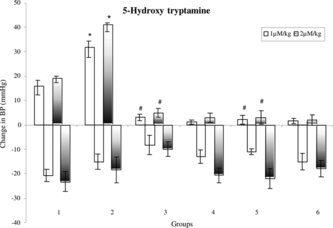
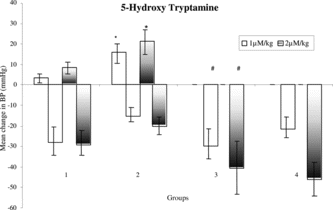
Effect of ME-100 on vascular reactivity to adrenaline, noradrenaline, and 5-hydroxy tryptamine in fructose hypertensive rats
Pressor responses to NA, Adr, and 5-HT were not altered significantly in case of ME-100-treated group as compared with control group. Pressor responses to NA and Adr except 5-HT were not significantly altered in case of fructose (10%) fed hypertensive rats as compared with control rats. Pressor responses to 5-HT were significantly reduced (p < 0.05) in case of fructose (10%) fed rats that received ME-100 for 6 weeks as compared with fructose (10%) fed hypertensive rats ().
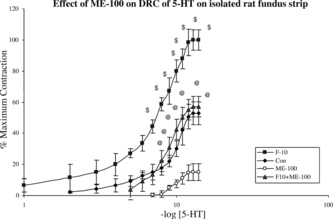
Effect of ME-100 on dose-response curve of 5-hydroxytryptamine on isolated rat stomach fundus strip of control and fructose hypertensive rats
Chronic administration of ME-100 for 6 weeks in fructose hypertensive rats shifted dose-response curve of 5-HT significantly (p < 0.001) to the right with suppression of maxima as compared with dose-response curve of fructose hypertensive rats on isolated stomach fundus strip. Chronic administration of ME-100 for 6 weeks in control rats shifted dose-response curve of 5-HT significantly to the right with suppression of maxima as compared with dose-response curve of control rats on isolated stomach fundus strip ().
Discussion
The aim of the current study was to investigate the antihypertensive property of methanol extract (ME) of fenugreek seeds and its methanol fraction (MF) in DOCA-salt and fructose hypertensive rats. The study showed that chronic administration of ME (30 mg kg−1 day−1, p.o.) and MF (15 mg kg−1 day−1, p.o.) containing alkaloid and saponin for 4 weeks significantly reduced blood pressure in unilateral nephrectomized DOCA-salt hypertensive rats. Chronic administration of ME (100 mg kg−1 day−1, p.o.) for 6 weeks significantly reduced blood pressure in fructose hypertensive rats. Thus, ME and MF of fenugreek seeds possess antihypertensive properties. Female rats were used in the DOCA-salt hypertensive model as they appear to be more susceptible to development of hypertension (Greenberg et al., Citation1973; Balaraman et al., Citation1989).
The hypertensive mechanism due to chronic administration of DOCA-salt has been well documented. DOCA-salt being a mineralocorticoid causes salt and water retention and by this contributes to the development of hypertension. Endothelin-1 (Matsumura et al., Citation1999), atrial natriuretic peptides (Ogawa et al., Citation1999), vasopressin (Bereck et al., Citation1982), and 5-hydroxytryptamine (Dawson et al., Citation1988) are involved in the pathogenesis of this type of hypertension.
The 5-HT2B receptor, first called atypical 5-HT receptor, was originally described as highly sensitive 5-HT receptor in the longitudinal smooth muscle of the rat stomach fundus (Vane, Citation1957). Recently, it has been hypothesized that in hypertension there is upregulation of 5-HT2B receptor in order to maintain an elevated blood pressure in rats made hypertensive by DOCA and NAME (Banes & Watts, Citation2002; Russel et al., Citation2002). It has been previously reported that part of this increase in arterial sensitivity to 5-HT is due to a change in the receptor population that mediates contraction to 5-HT under conditions of DOCA-salt hypertension. Specifically, Watts and colleagues (Watts, Citation1988; Watts et al., Citation1995Citation1996) presented pharmacological and molecular evidence that a 5-HT2A receptor population (ketanserin sensitive) primarily mediates contraction in arteries from normotensive rats, and a 5-HT2B receptor population (relatively ketanserin insensitive) primarily mediates arterial contraction in DOCA-salt hypertension. This “switch” is important because 5-HT possesses 300 to 1000 times higher affinity for the 5-HT2B receptor compared with the 5-HT2A receptor (Wainscott et al., Citation1993Citation1996); thus, lower concentrations of 5-HT are necessary to activate the 5-HT2B receptor. Moreover, this finding makes important the reexamination of hypertension with new pharmacological tools that block the 5-HT2B receptor, because the serotonergic antagonist most frequently tested has been ketanserin, and ketanserin possesses ∼ 1000 times lower affinity for the 5-HT2B receptor compared with the 5-HT2A receptor. LY-272015, a tetrahydro-β carboline recently developed as a 5-HT2B receptor antagonist (Cohen et al., Citation1996), has been shown to reduce blood pressure, suggesting that 5-HT2B receptors are endogenously upregulated in order to maintain an elevated blood pressure.
On the basis of these findings, we wished to test the putative 5-HT2B antagonistic property of ME and MF in DOCA-salt hypertensive rats. ME (30 mg kg−1 day−1, p.o.) and MF (15 mg kg−1 day−1, p.o.) were able to reduce blood pressure in DOCA-salt hypertensive rats but did not alter blood pressure in sham-operated control rats, implying an antihypertensive effect. ME and MF selectively blocked the rise of serotonin in hypertensive rats on vascular reactivity. This suggests that ME and MF act through influencing the serotonergic pathway in hypertensive rats, which is mediated by 5-HT2 receptors.
Further, the antihypertensive effect of ME and MF does not involve modulation of the renin-angiotensin system because the antihypertensive effect was observed in DOCA-salt hypertensive rats, a low renin model of experimental hypertension.
Currently, researchers are taking an interest in using a fructose-induced hypertension model, as it gives a clue about the role of dietary changes in hypertension, which has become an important factor of the modern lifestyle.
The mechanism of fructose-induced hypertension is still not clear. Recent studies have shown that a high-fructose diet is associated with increased blood pressure in rats (Bunnag et al., Citation1997; Dimo et al., Citation2001aCitationb; Juann et al., Citation1988). Several studies have demonstrated that chronic fructose feeding leads to insulin resistance, glucose intolerance, hyperinsulinemia, hyperglycemia, and hypertriglyceridemia in a relatively short time in normal rats (Zavaroni et al., Citation1980; Hwang et al., Citation1987; Erlich & Rosenthal, Citation1995). These metabolic changes lead to essential hypertension (Rosen et al., Citation1997). Hyperinsulinemia could activate the sympathetic system, which in turn could elevate the BP (Hwang et al., Citation1987). An impaired response to endothelium-dependent vasodilators in fructose-fed rats has also been demonstrated (Richey et al., Citation1998). A growing body of evidence indicates that locally generated vasoactive substances such as angiotensin-II (Ang-II) and nitric oxide (NO) are important determinants of the natural history of vascular disease. Recent evidence suggests that endothelial NO production could be decreased in fructose-fed rats at both renal (Nagai et al., Citation2002) and vascular levels (Shinozaki et al., Citation1999). Alterations in both endothelial production of NO and vascular smooth muscle cell (VSMC) growth could be associated with the initiation or progression of the atherosclerotic process and to vascular changes in hypertension.
In the current study, the effect of the methanol extract of fenugreek seed ME (100 mg kg−1 day−1, p.o.) was examined on hypertension induced by fructose (10%) given in drinking water. Male rats were used in this study as female rats do not develop hypertension or hyperinsulinemia upon fructose feeding except after ovariectomy, suggesting that female sex hormones may confer protection against the effects of a fructose diet (Galipeau et al., Citation2002). Parameters like systolic blood pressure and vascular reactivity to 5-HT were modified significantly by chronic administration of ME (100 mg kg−1 day−1, p.o.) in fructose hypertensive rats.
Results from vascular reactivity also support the serotonin antagonistic property of ME. The blockade of rise of BP in hypertensive rats, which is 5-HT2B receptor–mediated, gives a clue that ME may act through 5-HT2B receptors.
The sensitivity of rat fundus to 5-HT from fructose-induced hypertensive rats was increased as compared with that in control rats. Treatment with ME (100 mg kg−1 day−1, p.o.) in fructose hypertensive rats shifted the dose-response curve of 5-HT significantly to the right compared with fructose hypertensive rats, suggesting upregulated 5-HT2B receptors may be blocked. Similarly, treatment with ME (100 mg kg−1 day−1, p.o.) in control rats also shifted the dose-response curve of 5-HT toward the right indicating 5-HT2B antagonistic properties of the extract.
The data of our study suggest that the methanol extract of fenugreek seeds can play a major role in elucidating the mechanisms underlying the pathogenesis of fructose-fed hypertension as demonstrated by the beneficial effects on blood pressure.
Collectively, the results of our study suggest that 5-HT plays an important role in development of hypertension and the antihypertensive activity of methanol extract, and methanol fraction of fenugreek seeds may be partly due to 5-HT2B receptor antagonism.
References
- Al Meshal IA, Parmar NS, Tariq M, Aqeel AM (1985): Gastric anti-ulcer activity in rats of Trigonella foenum-graecum. (Hu-Lu-Pa). Fitoterapia 56: 232–235. [CSA]
- Balaraman R, Gulati OD, Bhatt JD, Bhatt SD, Rathod SP, Hemavati KG (1989): Cadmium induced hypertension in rats. Pharmacology 38: 226–234. [INFOTRIEVE], [CSA]
- Banes AK, Watts SW (2003): Arterial expression of 5-HT2B and 5-HT1B receptors during development of DOCA-salt hypertension. BMC Pharmacol 3: 12–19. [INFOTRIEVE], [CSA], [CROSSREF]
- Banes AK, Watts SW (2002): Upregulation of arterial serotonin 1B and 2B receptors in deoxycorticosterone acetate-salt hypertension. Hypertension 39: 394–398. [INFOTRIEVE], [CSA], [CROSSREF]
- Bereck KH, Barron KW, Webb RL, Brody MJ (1982): Vasopressin CNS interactions in the development of DOCA hypertension. Hypertension 4: 131–137. [CSA]
- Bunnag P, Hori MT, Ormsby B, Berger ME, Golub MS, Tuck ML (1997): Impaired in vivo. adrenergic responses in diet-induced hypertensive rats. Hypertension Res 20: 17–21. [CSA]
- Chopra RN (1982): In: Chopra IC, Honda KL, Kapur LD, eds., Chopra's Indigenous Drugs of India, Calcutta, New Delhi, Academic Publishers, p. 582.
- Cohen ML, Schenck KW, Mabry TE, Nelson DL, Audia JE (1996): LY272015, a potent selective and orally active 5-HT2B receptor antagonist. J Hypertens (Suppl): 131–144. [CSA]
- Dai S, McNeill JH (1995): Fructose induced hypertension in rats is concentration and duration dependent. J Pharmacol Toxicol Methods 33: 101–107. [INFOTRIEVE], [CSA], [CROSSREF]
- Dawson R Jr, Nagamhama S, Oparil S (1988): Central serotonergic alterations in deoxycorticosterone acetate/Nacl-induced hypertension. Neuropharmacology 27: 417–426. [INFOTRIEVE], [CSA], [CROSSREF]
- Dimo T, Azay J, Tan PV, Pellecuer J, Cros G, Bopelet M, Serrano JJ (2001a): Effects of the aqueous and methylene chloride extracts of Bidens pilosa. leaf on fructose-hypertensive rats. J Ethnopharmacol 76: 215–221. [INFOTRIEVE], [CSA], [CROSSREF]
- Dimo T, Rakotonirina A, Tan PV, Dongo E, Dongmo AB, Kamtchouing P, Azay J, Abegaz BM, Cros G, Ngadjui TB (2001b): Antihypertensive effects of Dorstenia psilurus. extract in fructose-fed hyperinsulinemic, hypertensive rats. Phytomedicine 8: 101–106. [INFOTRIEVE], [CSA], [CROSSREF]
- Erlich Y, Rosenthal T (1995): Effect of angiotensin-converting enzyme inhibitors on fructose induced hypertension and hyperinsulinaemia in rats. Clin Exp Pharmacol Physiol 22(Suppl): S347–llast.
- Galipeau D, Verma S, Mc Neill J (2002): Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am J Physiol Heart Circ Physiol 283: H2478–H2484. [INFOTRIEVE], [CSA]
- Ghosal S, Srivastava RS, Chatterjee DC, Dutta SK (1974): Extractives of Trigonella.-1 Fenugreekine, a new steroidal sapogenin-peptide ester of Trigonella foenum-graecum.. Phytochemistry 13: 2247–2251. [CSA], [CROSSREF]
- Goyal RK (1999): Practicals in Pharmacology, 2nd ed. Gujrat, B. S. Shah Prakashan, p. 48.
- Greenberg S, Heitz DC, Long JP (1973): Testosterone induced depression of adrenergic activity in the perfused canine hindlimb. Proc Soc Exp Biol Med 142: 883–888. [INFOTRIEVE], [CSA]
- Hwang IS, Ho H, Hoffman BB, Reaven GM (1987): Fructose-induced insulin resistance and hypertension in rats. Hypertension 10: 512–516. [INFOTRIEVE], [CSA]
- Jayaweera DM (1981): Medicinal Plant, Pt. 3. Srilanka, Peradenia, p. 255.
- Jha NK (2003): Trigonella foenum-graecum.: Fenugreek, Methi. Phytopharm 4: 3–15. [CSA]
- Juan CC, Fang VS, Hsu YP, Huang YJ, Hsia DB, Yu PC, Kwok CF, Ho LT (1988): Overexpression of vascular endothelin-1 and endothelin-A receptors in a fructose-induced hypertensive rat model. J Hyp 12: 1775–1782. [CSA]
- Kirtikar KR, Basu BD (1993): Indian Medicinal Plants, 2nd ed. vol. 1. Allahabad, Lalit Mohan Basu, pp. 19, 700.
- Matsumura Y, Hashimoto N, Taira S, Kuro T, Kitano R, Ohkita M, Opgenorth TJ, Takaoka M (1999): Different contributions of endothelin-A and endothelin-B receptors in the pathogenesis of deoxycorticosterone acetate-salt induced hypertension in rats. Hypertension 33: 759–765. [INFOTRIEVE], [CSA]
- Morton JF (1990): Mucilaginous plants and their uses in medicine. J Ethnopharmacol 29: 215–266. [CSA], [CROSSREF]
- Nagai Y, Nishio Y, Nakamura T, Maegawa H, Kikkawa R, Kashiwagi (2002): Amelioration of high fructose-induced metabolic derangements by activation of PPARalpha. Am J Physiol Endocrinol Metab 282: E1180–E1190. [INFOTRIEVE], [CSA]
- Nagawa M, Nasjletti A (1988): Plasma kinin concentration in deoxycorticosterone hypertension. Hypertension 11: 411–415. [CSA]
- Ogawa T, Linz W, Scholkens BA, deBold JA (1999): Variable renal natriuretic factor gene expression in hypertension. Hypertension 33: 1342–1347. [INFOTRIEVE], [CSA]
- Ribes G (1984): Effect of fenugreek seeds on endocrine pancreatic secretions in dogs. Ann Nutr Metab 28: 37–43. [INFOTRIEVE], [CSA]
- Richey JM, Si X, Halter JB, Webb RC (1998): Fructose perfusion in rat mesenteric arteries impairs endothelium-dependent vasodilation. Life Sci 62: PL55–PL62. [INFOTRIEVE], [CSA], [CROSSREF]
- Rosen P, Ohly P, Gleichmann H (1997): Experimental benefit of moxonidine on glucose metabolism and insulin secretion in the fructose-fed rat. J Hypertens (Suppl) 15: S31–S38. [CSA]
- Russell A, Banes A, Berlin H, Fink GD, Watts SW (2002): 5-HT2B receptor function is enhanced in N.ω- nitro-L-arginine hypertensive rats. J Pharmacol Exp Ther 303: 179–187. [INFOTRIEVE], [CSA], [CROSSREF]
- Selye H, Bois P (1957): The hormonal production of nephrosclerosis and periarteritis nodosa in the primate. Br Med J 1: 183–186. [INFOTRIEVE], [CSA]
- Shani J, Gold Schmied A, Ahronson Z, Sulman FG (1974): Hypoglycemic effect of Trigonella foenum-graecum. and Lupinus termis. (Leguminosae) seeds and their major alkaloids in alloxan diabetic and normal rats. Arch Int Pharmacodyn Ther 210: 27–36. [CSA]
- Sharma RD (1986): An evaluation of hypocholesterolaemic factor of fenugreek seeds (Trigonella foenum-graecum.) in rats. Nutr Rep Int 33: 669–677. [CSA]
- Shinozaki K, Kashiwagi A, Nishio Y, Okamura T, Yoshida Y, Masada M, Toda N, Kikkawa R (1999): Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2-imbalance in insulin-resistant rat aorta. Diabetes 48: 2437–2445. [INFOTRIEVE], [CSA]
- Suzuki M, Nomura C, Odaka H, Ikeda H (1997): Effect of an insulin sensitizer, pioglitazone, on hypertension in fructose drinking rats. Jpn J Pharmacol 74: 297–302. [INFOTRIEVE], [CSA]
- Vane JR (1957): A sensitive method for the assay of 5-hydroxytryptamine. Br J Pharmacol 12: 344–349. [CSA]
- Verma S, Bhanot S, Mc Neill JH (1994): Decreased vascular reactivity in metformin treated fructose hypertensive rats. J Pharmacol Exp Ther 271: 1334–1337. [INFOTRIEVE], [CSA]
- Vogel GH (2002): Drug Discovery and Evaluation, 2nd ed. Berlin, Heidelberg, Springer-Verlag, pp. 176.
- Wainscott D, Cohen ML, Schenck KW, Au JE, Nissen JS, Baez M, Kursar JD, Luca VL, Nelson DL (1993): Pharmacological Characteristics of the newly cloned rat 5-HT2F receptor. Mol Pharmacol 43: 419–426. [INFOTRIEVE], [CSA]
- Wainscott DB, Lucaites VL, Kursar JD, Baez M, Nelson DL (1996): Pharmacological characterization of the human 5-hydroxytryptamine2B receptor: Evidence for species differences. J Pharmacol Exp Ther 276: 720–727. [INFOTRIEVE], [CSA]
- Watts SW (1998): The development of enhanced arterial serotonergic hyperresponsiveness in mineralocorticoid hypertension. J Hypertens 16: 811–822. [INFOTRIEVE], [CSA], [CROSSREF]
- Watts SW, Gilbert L, Webb RC (1995): 5-Hydroxytryptamine2B receptor mediates contraction in the mesenteric artery of mineralocorticoid hypertensive rats. Hypertension 26: 1056–1059. [INFOTRIEVE], [CSA]
- Watts SW, Baez M, Webb RC (1996): The 5-hydroxytryptamine2B receptor and 5-HT receptor signal transduction in mesenteric arteries from deoxycorticosterone acetate-salt hypertension. J Pharmacol Exp Ther 277: 1103–1113. [INFOTRIEVE], [CSA]
- Zavaroni I, Sander S, Scott S, Reaven GM (1980): Effect of fructose feeding on insulin secretion and insulin action in the rat. Metabolism 29: 970–973. [INFOTRIEVE], [CSA], [CROSSREF]