Abstract
This research was undertaken to find the in vitro. inflammatory action of 5,7-dimethyloxy-4-p.-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin produced by Streptomyces aureofaciens. CMUAc130. We investigated the effects of 5,7-dimethyloxy-4-p.-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin not only on the formation of NO, prostaglandin E2 (PGE2), and tumor necrosis factor-α. (TNF-α.) but also on inducible nitric oxide synthase and cyclooxygenase-2 (COX-2) in lipopolysaccharide (LPS)-induced murine macrophage RAW 264.7 cells. The data obtained were consistent with the modulation of iNOS enzyme expression. A similar fashion was also observed when LPS-induced PGE2 release and COX-2 expression were tested. The significant inhibitory effects were shown in concentration-dependent manners. In addition, 5,7-dimethyloxy-4-p.-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin also mildly but significantly reduced the formation of TNF-α.. These findings support the application of 5,7-dimethyloxy-4-p.-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin as anti-inflammatory agents.
Introduction
The organism Streptomyces aureofaciens. CMUAc130 was isolated from the root tissue of Zingiber officinale. Rosc. (Zingiberaceae). It produced 5,7-dimethyloxy-4-p.-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin, (), which had antifungal activities against Colletotrichum musae. and Fusarium oxysporum., the causative agents of anthracnose of banana and wilt of Wheat, respectively (Taechowisan et al., Citation2005). In a course of searching for the anti-inflammatory agent from these compounds, we investigated nitric oxide (NO) inhibition in lipopolysaccharide (LPS) activated macrophage cells. NO is synthesized via the oxidation of l-arginine by a family of nitric oxide synthases (NOS) (Moncada et al., Citation1993), which are either constitutive (cNOS) or inducible (iNOS). A cNOS is Ca2+-dependent and releases small amounts of NO required for physiological functions (Bredt et al., Citation1990), whereas the other form, iNOS, is Ca2+-independent (Lowenstein et al., 1992) and is induced by LPS or proinflammatory cytokines such as TNF-α., IL-1β., and IFN-γ.. In practice, during inflammatory processes, large amounts of proinflammatory mediators, NO and prostaglandin E2 (PGE2) are generated by the iNOS and cyclooxygenase-2 (COX-2), respectively (Lee et al., Citation1992). TNF-α., a primary mediator in the septic circulation, has been known to induce iNOS. Cyclooxygenase (COX) is the enzyme that converts arachidonic acid to prostaglandins (PGs). Like NOS, COX has been found in two isoforms, and COX-2 is an inducible form responsible for the production of large amounts of proinflammatory PGs at the inflammatory site (Weisz et al., Citation1996). Therefore, an inhibitor of iNOS, COX, and TNF-α. may be effective as a therapeutic agent for inflammation. Although several phamacological effects of 4-arylcoumarins are reported, there have been no reports on the effects of 4-arylcoumarins on the anti-inflammatory role. Therefore, this communication will encourage an effort to search for new antiinflammatory 4-arylcoumarins.
Materials and Methods
Organisms and compounds
Streptomyces aureofaciens. CMUAc130 was isolated from the root tissues of Zingiber officinale. by the surface-sterilization technique (Taechowisan et al., Citation2003). Identification of the isolate to species level was based on morphological, cultural, physiological, and biochemical characteristics and also 16S rDNA gene sequencing as described by Taechowisan and Lumyong (Citation2003). Solid medium for sporulation used in this study was International Streptomyces. Project Medium 4 (ISP-4), and the liquid medium used for fermentation was ISP-2 (Shirling & Gottlieb, Citation1966). The large-scale fermentation, crude extraction, and purification of the compounds was carried on by the methods of Taechowisan (Taechowisan et al., Citation2005).
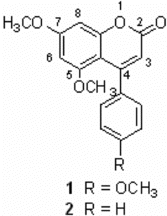
Structure elucidation of the compounds
The structures of the active compounds () have been identified using NMR and mass spectral data. The melting point of the compounds was determined on a Buchi-540 melting point apparatus. Optical rotations were measured on a Perkin-Elmer 241 polarimeter, IR spectra on a Perkin-Elmer 1 spectrometer, 1H and 13C NMR spectra on a Bruker DRX 500 spectrometer, and EI-MS and FAB-MS, respectively, on a Hewlett-Packard 5989 B and a Finnigan/Thermo Quest Mat 95 XL mass spectrometer.
Cell culture and sample treatment
RAW 264.7 murine macrophage cell line was obtained from the Korea Cell Line Bank (Seoul, Korea). These cells were grown at 37°C in DMEM medium supplement with 10% FBS, penicillin (100 units/mL), and streptomycin sulfate (100 µg/mL) in a humidified atmosphere of 5% CO2. Cells were incubated with 5,7-dimethyloxy-4-p.-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin at increasing concentrations and simulated with LPS 1 µ/mL for 24 h.
MTT assay for cell viability
Cytotoxicity studies were performed on a 96-well plate. RAW 264.7 cells were mechanically scraped and plated 2 × 105/well on 96-well plate containing 100 mL of DMEM medium with 10% FBS and incubated overnight. The 5,7-dimethyloxy-4-p.-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin were dissolved in dimethylsulfoxide (DMSO) for stock solution. The DMSO concentrations in all assays did not exceed 0.1%. Twenty-four hours after seeding, 100 µL new media or 5,7-dimethyloxy-4-p.-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin were added, and the plates were incubated for 24 h. Cells were washed once before adding 50 µL FBS-free medium containing 5 mg/mL MTT. After 4 h of inoculation at 37°C, the medium was discarded and the formazan blue, which formed in the cells, was dissolved in 50 µL DMSO. The optical density was measured at 540 nm. The concentration required for reducing the absorbance by 50% (IC50) compared with the control cells was determined.
Nitrite assay
Nitrite accumulation, an indicator of NO synthesis, was measured in the culture medium by Griess reaction (Green et al., Citation1982). Briefly, 100 µL of cell culture medium was mixed with 100 µL of Griess reagent [equal volumes of 1% (w/v) sulfanilamide in 5% (v/v) phosphoric acid and 0.1% (w/v) naphtylethylenediamine-HCl] and incubated at room temperature for 100 min, and then the absorbance at 550 nm was measured in a microplate reader. Fresh culture medium was used as the blank in all experiments. The amount of nitrite in the samples was calculated from a sodium nitrite standard curve freshly prepared in culture medium.
PGE2 and TNF-α assay
PGE2 and TNF-α. levels in macrophage culture medium were quantified by EIA kits according to the manufacturer's instructions.
Western blot assay
Cellular proteins were extracted from control and 5,7-dimethyloxy-4-p.-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin treated RAW 264.7 cells. The washed cell pellets were resuspended in lysis buffer (50 mM HEPES pH 7.0, 250 mM NaCl, 5 mM EDTA, 0.1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol, 5 mM NaF, 0.5 mM Na orthovanadate) containing 5 µg/mL each of leupeptin and aprotinin and incubated for 15 min at 4°C. Cell debris was removed by microcentrifugation, followed by quick freezing of the supernatants. Protein concentration was determined by BioRad protein assay reagent according to the manufacturer's instruction, and 40–50 µg of cellular proteins from treated and untreated cell extracts were electroblotted onto nitrocellulose membrane following separation on a 10% SDS-polyacrylamide gel electrophoresis. The immunoblot was incubated overnight with blocking solution (5% skim milk) at 4°C, followed by incubation for 4 h with a 1:500 dilution of monoclonal anti-iNOS and COX-2 antibody (Santa Cruz, CA, USA). Blots were washed two times with PBS and incubated with a 1:1000 dilution of horseradish peroxidase–conjugated goat anti-mouse IgG secondary antibody (Santa Cruz, CA, USA) for 1 h at room temperature. Blots were again washed three times in Tween 20/Tris-buffered saline (TTBS) and then developed by enhanced chemiluminescence (Amersham Life Science, Arlington Heights, IL, USA).
Data analysis
Data are reported as mean±SEM values of three independent determinations. All experiments were done at least three times, each time with three or more independent observations. Statistical analysis was performed by Student's t.-test.
Results and Discussion
We have studied the physiological roles of 5,7-dimethyloxy-4-p.-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin for the development of biologically active substances (Taechowisan et al., Citation2005). Although there are many coumarin derivatives that have special ability to scavenge reactive oxygen species–free radicals, such as hydroxyl radicals, superoxide radicals, or hypochlorous acid, and to influence processes involving free-radical injury (Mora et al., Citation1990), they have also been found to inhibit lipid peroxidation and to possess vasorelaxant (Hoult & Paya, Citation1996) and anticoagulant activities (Visser et al., Citation2000) and anti-inflammatory/antioxidant activity (Fylaktakidou et al., Citation2004; Kontogiorgis & Hadjipavlou, Citation2004; Nicolaides et al., Citation2004). Moreover, they have been used as inhibitors of lipoxygenase and cyclooxygenase in the arachidonic acid cascade (Kimura et al., Citation1985; Craven et al., Citation1986) of serine proteases (Doucet et al., Citation1999; Pochet et al., Citation2000) and of tyrosine kinase (Hoult & Paya, Citation1996). We selected 5,7-dimethyloxy-4-p.-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin with a potent NO inhibitory action from the crude extract.
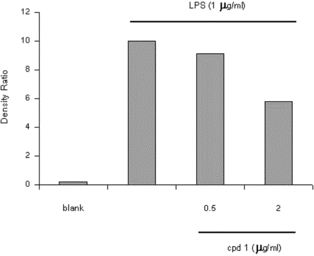
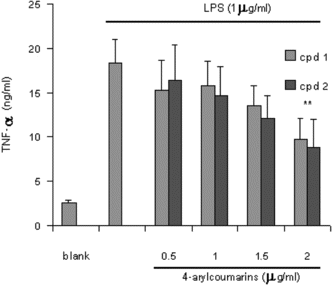
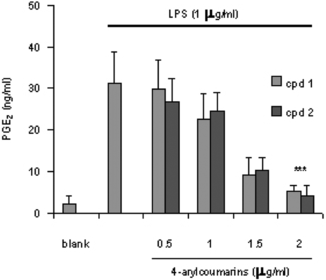
It is well-known that macrophages play a crucial role in both nonspecific and acquired immune responses, and macrophage activation by LPS leads to a functionally diverse series of responses including the production of proinflammatory cytokines (IL-1, TNF-α., and IL-6), the activation of phospholipase A2 producing lipid metabolites of arachidonic acid such as PGs, and NO production. As shown in , the LPS (1 µg/mL) highly induced NO2 production in murine macrophage RAW 264.7 cells. Treatment of 5,7-dimethyloxy-4-p.-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin (0.5–2.0 µg/mL) prevented NO production in dose-dependent fashion and cell viability was observed to be more than 92% by the MTT assay. Results from Western blotting analysis further indicated that LPS-induced expression in RAW 264.7 macrophages was significantly reduced (). The enzyme iNOS is responsible for long-lasting NO production and it is strikingly induced by LPS as shown in this study. Therefore, these results suggest that inhibition of LPS-induced NO production is due to iNOS gene expression. Mechanism of various anti-flammatory action is at least shared by the inhibition of prostaglandin synthesis, which is mediated by COX. This exists in two isoforms, COX-1 and 2, each with distinct expression pattern in various cell types. COX-1 has been suggested to provide a physiologic level of prostaglandins for normal platelet, stomach, and kidney function. In contrast, COX-2 has been found to be highly induced at inflammatory sites in animals as well as patients with inflammatory diseases (Masferrer et al., Citation1994; Seibert et al., Citation1994), and it is considered to be responsible for proinflammatory prostaglandin formation. In addition to inhibition of NO release and iNOS induction, 5,7-dimethyloxy-4-p.-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin also significantly inhibited PGE2 production and COX-2 gene expression in LPS-treated RAW 264.7 macrophages ( and ). Proinflammatory cytokines such as TNF-α., IL-1, and IL-6 have been shown to control inflammation in vitro. as well as in vivo. (Harada et al., Citation1994; Feldmann et al., Citation1996), and these cytokines are thought to be interlinked in a cascade, being produced serially by macrophages during an inflammatory response. Furthermore, the development of hyperalgesic states during inflammation is thought to be mediated by proinflammatory cytokines (Watkins et al., Citation1995). Therefore, we investigated whether 5,7-dimethyloxy-4-p.-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin would possibly influence the formation of TNF-α. in an in vitro. model. The 5,7-dimethyloxy-4-p.-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin mildly affected the production of TNF-α. ().
Figure 2 Evaluation of nitrite production by RAW 264.7 cells stimulated for 24 h with LPS alone or in combination with increasing concentrations (0.5–2 µg/mL) of 5,7-dimethoxy-4-p.-methoxylphenylcoumarin and (cpd 1) and 5,7-dimethoxy-4-phenylcoumarin (cpd 2). The values are the means of at least 3 determinations ±SD. Probability levels (Student's t.-test): ***p < 0.001 versus LPS-treated group.
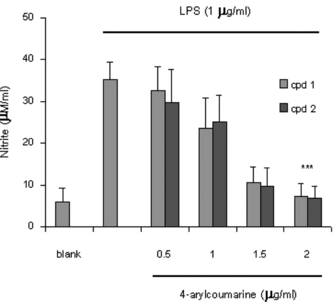
Figure 3 Effect of 5,7-dimethoxy-4-p.-methoxylphenylcoumarin (cpd 1) on iNOS production by LPS-induced RAW 264.7 macrophage for 24 h.
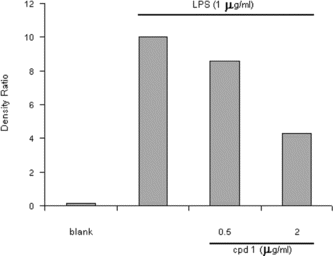
Figure 4 Effect of 4-arylcoumarins on PGE2 production in LPS-induced RAW 264.7 macrophage for 24 h. The values are the means of at least three determinations± SD. Probability level (Student's t.-test): ***p < 0.001 versus LPS-treated group.
Acknowledgment
We are grateful to Prof. Pittaya Tantiwachwuttikul for isolation the pure compounds. This research project was funded by Thailand Research Fund MRG4980137.
References
- Bredt DS, Snyder SH (1990): Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA 87: 682–685. [INFOTRIEVE], [CSA]
- Craven PA, Pfanstiel J, DeRubertis FR (1986): Role of reactive oxygen in bile salt stimulation of colonic epithelial proliferation. J Clin Invest 77: 850–859. [INFOTRIEVE], [CSA]
- Doucet C, Pochet L, Thierry N, Pirotte B, Delarge J, Reboud-Ravaux M (1999): 6-Substituted 2-oxo-2H-1-benzopyran-3-carboxylic acid as a core structure for specific inhibitors of human leukocyte elastase. J Med Chem 42: 4161–4171. [INFOTRIEVE], [CSA], [CROSSREF]
- Feldmann M, Brennan FM, Maini RN (1996): Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 14: 397–440. [INFOTRIEVE], [CSA], [CROSSREF]
- Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN (2004): Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr Pharm Des 10: 3813–3833. [INFOTRIEVE], [CSA], [CROSSREF]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982): Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126: 131–138. [INFOTRIEVE], [CSA], [CROSSREF]
- Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K (1994): Essentail involoement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol 56: 559–564. [INFOTRIEVE], [CSA]
- Hoult JR, Paya M (1996): Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential. Gen Pharmacol 27: 713–722. [INFOTRIEVE], [CSA]
- Kimura Y, Okuda H, Arichi S, Baba K, Kozawa M (1985): Inhibition of the formation of 5-hydroxy-6,8,11,14-eicosatetraenoic acid from arachidonic acid in polymorphonuclear leukocytes by various coumarins. Biochem Biophys Acta 834: 224–229. [INFOTRIEVE], [CSA]
- Kontogiorgis CA, Hadjipavlou-Litina DJ (2004): Synthesis and biological evaluation of novel coumarin derivatives with a 7-azomethine linkage. Bioorg Med Chem Lett 14: 611–614. [INFOTRIEVE], [CSA], [CROSSREF]
- Lee SH, Soyoola E, Chanmugan P, Hart S, Sun W, Zhong H, Liou S, Simmons D, Hwang D (1992): Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem 267: 25934–25938. [INFOTRIEVE], [CSA]
- Masferrer JL, Zweifel BS, Manning PT, Hauser SD, Leahy KM, Smith WG, Isakson PC, Seibert K (1994): Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci USA 91: 3228–3232. [INFOTRIEVE], [CSA], [CROSSREF]
- Monacada S, Higgs A (1993): The L-arginine-nitric oxide pathway. N Engl Med 329: 2002–2012. [CSA], [CROSSREF]
- Mora A, Paya M, Rios JL, Alcaraz MJ (1990): Structure-activity relationships of polymethoxyflavones and other flavonoids as inhibitors of non-enzymic lipid peroxidation. Biochem Pharmacol 40: 793–797. [INFOTRIEVE], [CSA], [CROSSREF]
- Nicolaides DN, Gautam DR, Litinas KE, Hadjipavlou-Litina DJ, Fylaktakidou KC (2004): Synthesis and evaluation of the antioxidant and anti inflammatory activities of some benzo[l]khellactone derivatives and analogues. Eur J Med Chem 39: 323–332. [INFOTRIEVE], [CSA], [CROSSREF]
- Pochet L, Doucet C, Dive G, Wouters J, Masereel B, Reboud-Ravaux M, Pirotte B (2000): Coumarinic derivatives as mechanism-based inhibitors of alpha-chymotrypsin and human leukocyte elastase. Bioorg Med Chem 8: 1489–1501. [INFOTRIEVE], [CSA], [CROSSREF]
- Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, Lee L, Isakson P (1994): Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA 91: 12013–12017. [INFOTRIEVE], [CSA], [CROSSREF]
- Shirling EB, Gottlieb D (1966): Methods for characterization of Streptomyces. species. Int J Syst Bacteriol 16: 313–340. [CSA]
- Taechowisan T, Lumyong S (2003): Activity of endophytics actinomycetes from roots of Zingiber officinale. and Alpinia galanga. against phytopathogenic fungi. Ann Microbiol 53: 291–298. [CSA]
- Taechowisan T, Peberdy JF, Lumyong S (2003): Isolation of endophytic actinomycetes from selected plants and their antifungal activity. World J Microbiol Biotechnol 19: 381–385. [CSA], [CROSSREF]
- Taechowisan T, Lu C, Shen Y, Lumyong S (2005): Secondary metabolites from endophytic Streptomyces aureofaciens. CMUAc130 and their antifungal activity. Microbiol 151: 1691–1695. [CSA], [CROSSREF]
- Visser LE, Trienekens PH, De Smet PA, Vulto AG, Hofman A, van Duijn CM, Stricker BH (2000): Patients with an ApoE epsilon4 allele require lower doses of coumarin anticoagulants. Pharmacogenet Genomics 15: 69–74. [CSA]
- Watkins LR, Maier SF, Goehler LE (1995): Immune activation: The role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain 63: 289–302. [INFOTRIEVE], [CSA], [CROSSREF]
- Weisz A, Cicatiello L, Esumi H (1996): Regulation of the mouse inducible-type nitric oxide synthase gene promoter by interferon-gamma, bacterial lipopolysaccharide and NG-monomethyl-L-arginine. Biochem J 316: 209–215. [INFOTRIEVE], [CSA]