Abstract
The role of free radicals in the etiology and development of a wide range of clinical disorders has continued to fuel the suggestion that phenolic antioxidants can offer a realistic promise to reduce the incidence of a number of pathologies involving oxidative stress. In this study, the total phenol, flavonoid, and proanthocyanidin contents of the Mauritian medicinal plants Crinum mauritianum. Lodd. (Asteraceae), Gaertnera psychotroides. DC (Rubiaceae), Psidia terebinthina. A.J. Scott (Asteraceae), and Tylophora coriacea. Marais. (Monimiaceae) were assessed and contrasted with their antioxidant potential. The antioxidant propensity was evaluated by the ability of the extracts to scavenge hypochlorous acid and hydroxyl radical and the ABTS⋅ + radical including their abilities to inhibit microsomal lipid peroxidation. The endemic plants Badula multiflora. A. DC. (Myrsinaceae), Croton vaughanii. L. (Euphorbiaceae), Erythroxylum macrocarpum. Lam. (Erythroxylaceae), Ochna mauritiana. Lam. (Ochnaceae), Tambourissa cordifolia. Lorence. (Monimiaceae), and Turraea rigida. Vent. (Meliaceae) were similarly investigated. Badula multiflora. and Erythoxylum macrocarpum. showed highest antioxidant activity in the TEAC and FRAP assay. Badula multiflora., Ochna mauritiana., and Gaertnera psychotroides. were very potent scavengers of hypochlorous acid and inhibited microsomal lipid peroxidation induced by 2,2′-azobis.(2-amidinopropane) hydrochloride (AAPH), suggesting that the inhibition was intrinsically linked to peroxyl radical scavenging. The antioxidant activity of Gaertnera psychotroides., Tylophora coriacea., Psidia terebinthina., and Crinum mauritianum. may account for the therapeutic effects of these extracts, in particular, in conditions characterized by inflammation and oxidative mechanisms. While these polyphenolic-rich endemics are good sources of natural prophylactic antioxidants, there is an urgent need for sustainable conservation programs for their protection.
Introduction
Considerable attention has been focused on plant resources worldwide as a potential source of pharmacologically active compounds. The African continent is particularly rich in species diversity, and it has been reported that southern Africa contains almost 10% of the world's flora with an estimated 23,404 vascular plant species (Arnold & De Wet, Citation1993; Cowling & Hilton-Taylor, Citation1994). A diversified and unique flora also characterizes Mauritius, a tropical island of volcanic origin in the Indian Ocean, which is considered to be part of the African continent. The island of Mauritius forms part of the Mascarene archipelago that consists of several outlying islands of the Indian Ocean, namely, Réunion, Rodrigues, Agalega, and Tromelin. Oceanic islands have been widely discussed for their rich diversity and high degree of endemism, particularly because they are far from continental masses, thus providing different sets of conditions conducive for speciation. It has been reported that 45% of the flora are strict endemics and include 311 taxa (Guého, Citation1988). This level of endemicity far exceeds estimates from Réunion and Rodrigues islands, both with 35%, and other African countries, mainly Namibia (17%), Zambia (4.4%), Zimbabwe (2.1%), and Bostwana (0.8%) (Guého, Citation1988; Maggs et al., Citation1998). The sustained use of flora for phytotherapy dates from time immemorial, and the use of medicinal plants play a dominant therapeutic role in the treatment of human ailments in both developing and developed countries. A wide number of the Mauritian endemics have been mentioned in the traditional pharmacopoeia for their medicinal uses against a wide range of physical ailments such as bronchitis, diabetes, asthma, dysentery, and inflammatory diseases (Gurib-Fakim, Citation1991; Bahorun et al., Citation2003).
Literature data has suggested that the therapeutic potential of medicinal plants lies in the presence of secondary metabolites including alkaloids, terpenoids, and polyphenols (Levêque et al., Citation1996). Plant polyphenols, in particular, have been greatly acknowledged for their biological and therapeutic activities, including antimicrobial, anti-inflammatory, vasodilatory, expectorant, antimutagenic, and antioxidant properties (McGregor et al., Citation1999; Spignoli, Citation2000; Soobrattee et al., Citation2005). In addition, the regulatory activity of phenolic antioxidants on cell signal transduction pathways, mainly, the MAPK and JNK kinases, and transcription factors, mainly, NF-κB, c-Jun, c-myc, and c-fos, have been highly emphasized as a strategy for reducing the incidence of diseases induced by reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Owuor & Kong, Citation2002; Hou et al., Citation2004). The prophylactic effects of phenolic antioxidants stem mainly from several epidemiologic studies and their ability to arrest the onset of chronic degenerative diseases in animal models and cell cultures (Kris-Etherton et al., Citation2002; Cao et al., Citation2004; Dijsselbloem et al., Citation2004; Bahorun et al., Citation2003). Literature data abounds in examples where phenolic compounds are the bioactive components responsible for the prophylactic benefits of traditionally used plants sources (Spignoli, Citation2000; Ishige et al., Citation2001; Ivanova et al., Citation2005). For instance, isoflavones (genistein, daidzein, biochanin) from soybean, also known as phytoestrogens, are applied in hormone replacement therapy (Dijsselbloem et al., Citation2004), and these phytochemicals also have applications in the prevention of atherosclerosis (Cassidy et al., Citation2003). Epigallocatechin gallate (-)-EGCG, the major antioxidative component of green tea, has been reported for growth inhibitory effects in cancer cells, which appears to be mediated by the induction of apoptosis, the arrest of a specific phase of the cell cycle, the inhibition of aberrant arachidonic acid metabolism, and the inhibition of proteasome activities (Fujiki et al., Citation2003; Hou et al., Citation2004).
Mauritian flora contains several endemic plants that are potential sources of phenolic compounds with antioxidant indications. Studies by Neergheen et al. (Citation2006) have shown that Syzygium commersonii, Syzygium glomeratum, syzygium mauritianum, syzygium venosum, Eugenia orbiculata, Eugenia elleptica, Eugenia tinifolia, Myonima obovata, Myonima nitens, Fernelia buxifolis and monimiastrum acutisepalum., contain significant levels of phenolic ompounds and thus can be suitable sources of natural antioxidant. In an extension of this work, other endemic plants () were assessed for their contents of phenolic antioxidant compounds. Notably, Crinum mauritianum, Gaertnera psychotroides, Psidia terebinthina, and Tylophora coriacea., have been discussed for their traditional uses (Tabel 1). The major impetus of this study involves the declining number of these species and the subsequent loss of potential sources of prophylactic agents. Considering the uniqueness of our flora and their polyphenolic richness and antioxidant propensities, there is an urge to reinforce existing conservation strategies to safeguard the threatened endemics with medicinal and potential therapeutic uses. This paper provides additional justification for the development of a sustained policy towards conservation.
Table 1.. List of plant species used in this study; traditional uses and status of species population are included.a.
Materials and Methods
Plant material
Mature leaves of Badula multiflora. A. DC., Erythroxylum macrocarpum. Lam., Ochna mauritiana. Lam., Gaertnera psychotroides. DC., Tambourissa cordifolia. Lorence, Croton vaughanii. L., Turraea rigida. Vent., Crinium mauritianum. Lodd., Tylophora coriacea. Marais, and Psidia terebinthina. A.J. Scott were obtained from Brise Fer forest, Le Mondrain natural reserve, and Le Pétrin conservation management area (center of Mauritius). The leaf specimens were harvested in 2002, immediately weighed, and kept at − 20°C prior to extraction. The leaf specimens were authenticated by the Mauritius Sugar Industry Research Institute, and voucher specimens were deposited at the Department of Biosciences, Faculty of Science, University of Mauritius.
Extraction
Leaves (50 g) were extracted first with acetone/water (70/30 v/v) (2 × 400 mL) and finally with pure methanol (3 × 400 mL). Filtrates were concentrated in vacuo., and the resulting aqueous extract was washed with dichloromethane to remove lipids before being freeze-dried. Part of the freeze-dried extract (corresponding with 1 g) fresh weight (FW) was dissolved in water for antioxidant assays, this solution being adjusted at a final 1/2 plant fresh weight/volume ratio. An aliquot of the freeze-dried extract (corresponding with 1 g FW) was dissolved in absolute methanol for storage at − 20°C for subsequent analyses, this solution being adjusted at a final 1/5 leaves fresh weight/volume ratio.
Determination of total phenol content
Total phenol content was determined by the method adapted from Singleton and Rossi (Citation1965) using the Folin-Ciocalteu reagent. To each tube, 0.25 mL of the extract was added followed by 3.75 mL of distilled water and 0.25 mL of Folin-Ciocalteu reagent. After 3 min, 20% sodium carbonate was added. The tubes were capped, mixed thoroughly, and heated at 40°C for 40 min. The blue coloration was read at 685 nm against a blank standard. Results were expressed in mg of gallic acid/g fresh weight of plant material.
Determination of total proanthocyanidin content
The HCl/butanol assay adapted from Porter et al., (Citation1986) was used to quantify the total amount of proanthocyanidins. To each tube, 0.25 mL of the methanol extract was added, followed by 3 mL of butanol/HCl solution and 0.1 mL of NH4Fe(SO4)2·12H2O in 2 M HCl. The tubes were capped and incubated for 40 min at 95°C. After cooling in the dark, the red coloration was then read at 550 nm against a blank standard. The amount of proanthocyanidins was expressed in mg of cyanidin chloride/g fresh weight.
Determination of total flavonoid content
The AlCl3 method (Lamaison & Carnet, Citation1990) was used for the quantification of the total flavonoid content of methanol plant extracts. AlCl3·6H2O (1.5 mL of 2%) was added to equal volumes of the extract. The mixture was shaken and the absorbance was read at 440 nm. Flavonoid contents were expressed in mg quercetin equivalent/g fresh weight.
Trolox equivalent antioxidant capacity (TEAC)
The ABTS/MnO2 method (Campos & Lissi, Citation1997) was used to evaluate the ability of the plant extracts to scavenge the preformed radical cation ABTS⋅ +. The ABTS⋅ + radical was generated by a reaction between ABTS (0.5 mM) and activated MnO2 (1 mM) in phosphate buffer (0.1 M). To 3 mL of the ABTS⋅ +solution, 0.5 mL of plant extracts was added, and the decay in absorbance was followed for 15 min at 734 nm. TEAC values were expressed in µmol trolox equivalent/g fresh weight for the plant extracts.
Ferric reducing antioxidant power (FRAP)
The ferric reducing antioxidant power (FRAP) assay gives an indication of the reducing ability of the plant extract. This method was adapted from Benzie and Strain (Citation1996). FRAP reagent was freshly prepared by mixing together 10 mM 2,4,6-tripyridyl triazine (TPTZ) and 20 mM ferric chloride in 0.25 M acetate buffer, pH 3.6. Plant sample (100 µL) was added to 300 µL of water followed by 3 mL of FRAP reagent. The absorbance was read at 593 nm after 4 min incubation at ambient temperature against a blank of distilled water. A calibration curve of ferrous sulfate (100–1000 µmol/L) was used, and results were expressed in µmol Fe (II)/g fresh weight of plant material.
Hypochlorous scavenging assay
The ability of the plant extract to scavenge hypochlorous acid (HOCl) was assessed. HOCl was prepared by adjusting the pH of a 1% (v/v) solution of NaOCl to 6.2 with dilute sulfuric acid. The working concentration of the stock solution was determined spectrophotometrically by measuring its absorbance at 235 nm and applying a molar extinction coefficient of 100 M−1 cm−1 (Weiss et al., Citation1982). The reaction mixture contained taurine (10 mM), HOCl (1 mM), plant extract (variable concentrations), phosphate saline buffer (pH 7.4) in a final volume of 1 mL. The solution was mixed thoroughly and incubated for 10 min at ambient temperature followed by the addition of 10 µL of potassium iodide. A yellow coloration was developed, and the absorbance was read at 350 nm. The results were expressed as IC50 values (mg FW/mL).
Deoxyribose assay
The hydroxyl radical scavenging potential of the extracts was determined using the deoxyribose assay (Halliwell et al., Citation1987; Aruoma, Citation1994). The reacting mixture contained in a final volume of 1 mL the following reagents: 200 µL KH2PO4-KOH (100 mM), 200 µL deoxyribose (15 mM), 200 µL FeCl3 (500 µM), 100 µL EDTA (1 mM), 100 µL sample, 100 µL H2O2 (10 mM), and 100 µL ascorbic acid (1 mM). Reaction mixtures were incubated at 37°C for 1 h.
At the end of the incubation period, 1 mL 1% (w/v) thiobarbituric acid (TBA) was added to each mixture followed by the addition of 1 mL 2.8% (w/v) trichloroacetic acid (TCA). The solutions were heated in a water bath at 80°C for 20 min to develop the pink-colored MDA-(TBA)2 adduct. As turbidity was encountered, the MDA-(TBA)2 chromogen was extracted into 2 mL of butanol and its absorbance measured at 532 nm. Results were expressed as the percentage inhibition of deoxyribose degradation/g fresh weight of plant material.
Preparation of microsomes
Beef liver microsomes were prepared by tissue homogenization with ice-cold 0.25 M sucrose–10 mM Tris buffer, pH 7.4, with 1 mM EDTA. Microsomal fractions were isolated by removal of the nuclear fractions at 1500 rpm for 5 min and removal of mitochondrial fraction at 5000 rpm for 15 min. The supernatant was then centrifuged in a Beckman Optima ultracentrifuge (Beckman, USA) at 100,000 × g. for 60 min at 4°C. The pellet was then washed in 0.25 M NaCl, pH 7.5. The membranes were suspended in saline (0.25 M, pH 7.5) and stored in aliquots at − 20°C. Microsomal protein was determined by the Lowry-Folin method (Lowry et al., Citation1951).
2, 2′-Azobis.(2-amidinopropane) hydrochloride (AAPH)-induced lipid peroxidation
The reaction mixture contained in a final volume of 0.8 mL the following reagents: 200 µL of beef liver microsomes (0.5 mg/mL microsomal protein final concentration) suspended in 0.1 M potassium phosphate buffer, pH 7.5, 400 µL sample, and 200 µL AAPH (20 mM) to initiate peroxidation. The mixture was incubated at 37°C for 1 h and the solution gently shaken at 10-min intervals. After incubation, 1.6 mL TCA-TBA-HCl stock solution (15% w/v trichloroacetic acid, 0.375% w/v thiobarbituric acid, 0.25_M HCl) was added. The solution was heated in a boiling water bath for 15 min. After cooling, the precipitate was removed by centrifugation and the absorbance of the resulting supernatant measured at 532 nm.
Statistical analysis
Results are expressed as mean value±standard deviation (n = 3). Simple regression analysis was performed to calculate the dose-response relationship of standard solutions used for calibration as well as for the test samples. Linear regression analysis was performed, quoting the correlation coefficient r.xy..
Results
Phenolic content of the plant extracts
The total phenol content of the endemic plant extracts measured by the Folin-Ciocalteu method ranged between 1 and 78 mg gallic acid/g FW. The highest phenol content was observed in Badula multiflora. (78 mg/g FW). Erythroxylum macrocarpum., Ochna mauritiana., and Gaertnera psychotroides. also had elevated levels of phenolics with 40, 34, and 32 mg/g FW, respectively. Moderate levels of phenolics were recorded in Tambourissa cordifolia., Croton vaughanii., and Turraea rigida., whereas Crinium mauritianum. was very poor in phenolics with minimal levels detected under this experimental condition (1.21 mg/g FW). The set of data is presented in .
Figure 1 Total phenol, total proanthocyanidin, and total flavonoid content of some highly threatened Mauritian endemic plant extracts. Values are expressed in mg/g fresh weight.
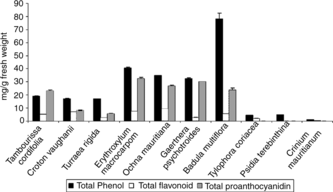
The proanthocyanidin content ranged from 6 to 37 mg/g FW with the highest level measured in E. macrocarpum., G. psychotroides., O. mauritiana., B. multiflora., and T. cordifolia. had appreciable levels of proanthocyanidins: 30±0.13, 30±0.63, 24±1.27, and 23±0.8 mg/g FW, respectively. Amounts less than 10 mg/g FW were measured in C. vaughanii. and T. rigida..
The flavonoid content was within the range of 0.1 to 9.3 mg quercetin equivalent/g FW. O. mauritiana. was shown to contain the highest level (9±0.18 mg/g FW) followed by E. macrocarpum. (7±0.12 mg/g FW) and C. vaughanii. (7±0.16 mg/g FW). Very low amounts were measured in P. terebinthina. and C. mauritianum..
Antioxidant activities of the plant extracts
The free radical scavenging abilities of the plant extracts were evaluated by monitoring the reduction of the ABTS⋅ + radical in the TEAC system and by measuring the scavenging potency toward hypochlorous acid, hydroxyl and peroxyl radicals in the HOCl scavenging, deoxyribose, and lipid peroxidation assays, respectively. The plant extracts showed high ABTS⋅ + radical scavenging with activities ranging from 3 to 849 µmol trolox equivalent/g FW. B. multiflora. had the highest measurable activity (849 µmol/g FW), and moderate TEAC values were observed for E. macrocarpum. (441 µmol/g FW), G. psychotroides. (358 µmol/g FW), and O. mauritiana. (302 µmol/g FW). The antioxidant hierarchy observed in the TEAC system was B. multiflora. > E. macrocarpum. > G. psychotroides. > O. mauritiana. > T. cordifolia. > T. rigida. > C. vaughanii. > T. coriacea. > P. terebinthina. > C. mauritianum.. C. mauritianum. extract exhibited a very low scavenging effect.
A similar pattern of activity to TEAC activity was obtained for the plant extracts in the ferric reducing assay. The ferric reducing activity ranged between 6 and 923 µmol/g FW. B. multiflora. and E. macrocarpum. showed high ferric reducing power with FRAP values of 923 and 517 µmol/g FW, respectively, whereas C. mauritianum. had the least measured activity. The endemic plant extracts were also powerful scavengers of hypochlorous acid. The concentration of extracts able to scavenge HOCl acid by 50% (IC50) under the experimental conditions was found to be in the following order: B. multiflora. > E. macrocarpum. > C. vaughanii. ≈ O. mauritiana. > T. cordifolia. > G. psychotroides. > T. rigida. > T. coriacea. ≈ P. terebinthina. > C. mauritianum.. B. multiflora. and E. macrocarpum. were strong HOCl scavengers with IC50 values of 0.43 and 0.89 mg FW/mL. C. vaughanii., O. mauritiana., T. cordifolia., G. psychotroides., and T. rigida. were also very effective scavengers. P. terebinthina. and T. coriacea. had comparable activity in the HOCl assay and showed relatively weaker activities (IC50 = 13 mg FW/mL).
The ability to inhibit lipid peroxidation initiated by the water-soluble AAPH initiator was assessed using beef liver microsomes as a lipid source. The latter undergo rapid nonenzymatic peroxidation when incubated in the presence of AAPH, which readily decomposes into peroxyl radicals. B. multiflora., O. mauritiana., G. psychotroides., and E. macrocarpum. were effective inhibitors of lipid peroxidation at low concentrations (). The calculated IC50 values obtained from the plots of concentration-dependent inhibition of oxidation are given in . No activity was measured under the experimental condition for C. mauritianum. extract thereby indicating that much higher concentrations of the extract would be required to inhibit microsome lipid peroxidation.
Figure 2 Inhibition of AAPH-induced microsome peroxidation by B. multiflora. and O. mauritiana. plant extracts. Concentrations yielding 50% inhibition for B. multiflora. and O. mauritiana. were 0.09 and 0.15 mg FW/mL, respectively.
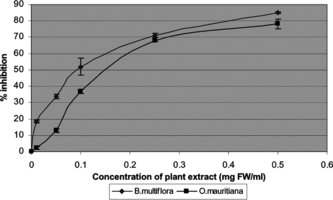
Table 2.. Antioxidant propensities of the plant extracts.
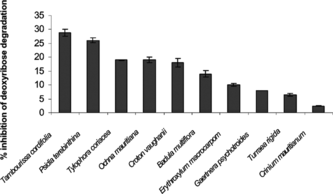
A mixture of FeCl3-EDTA, ascorbate, and H2O2 at pH 7.4 generates hydroxyl radicals, which can be measured by their ability to degrade the sugar deoxyribose into products that generate a pink chromogen on heating with thiobarbituric acid at low pH (Aruoma, Citation1994). The activity order measured in the deoxyribose assay was in the following decreasing order: T. cordifolia. > P. terebinthina. > O. mauritiana. ≈ T. coriacea. > C. vaughanii. > B. multiflora. > E. macrocarpum. > G. psychotroides. > T. rigida. > C. mauritianum. (). Crinum mauritianum. was a very weak inhibitor of deoxyribose damage by hydroxyl radicals generated by the Fenton system. The maximum activity was observed with T. cordifolia. plant extract with a 29% inhibition of damage followed by P. terebinthina. (26%).
Discussion
The high degree of endemism reflects the uniqueness of the Mauritian flora, and consequently several of these plant species may be of significant economic importance and medicinal use. This unique flora has resulted from colonization and adaptive radiation of plant species from different sources. Seventy percent of the phanerogam genera seemed to have been derived from Madagascar and the mainland Africa, 8% from Asia, 12% of pan Indo-Pacific origin, and 8% are endemic. Furthermore, because more than 50% of all modern clinical drugs are of natural product origin (Farombi, Citation2003), it can be speculated that the Mauritian endemic flora can be an interesting source of bioactive compounds. Thus, it is important for prioritizing sites for conservation in order to protect the dwindling number of the endemic plant species. This prompted the evaluation of the antioxidant efficacy in relation to the phenolic contents of some selected Mauritian endemic plants with medicinal value.
The Folin-Ciocalteu method indicated high phenolic content in some of the endemic plant extracts studied, in particular B. multiflora., E. macrocarpum., O. mauritiana., and G. psychotroides.. Substantial levels of proanthocyanidins and flavonoids have also been observed in these plant extracts, and the data are indicative of the polyphenolic richness of the Mauritian endemic flora. Tropical plants are tolerant of high levels of environmental stress induced by ultraviolet radiation and pollutants including smoke, and this may explain the high levels of phenolic compounds in the endemic plant species studied. Literature data abounds in examples where plant extracts rich in phenolic compounds and plant-derived constituents are used for their prophylactic benefits (Katsube et al., Citation2004; Škerget et al., Citation2005). For instance, some edible plants from Japan, particularly Mallotus japonicus. (“akamegashiwa”), Camellia sinensis. (green tea), Ligustrum japonium. (Japanese privet), and Diospyros kaki. (astringent persimmon), contain high levels of phenolic compounds, which have been suggested to account for the medicinal properties of these plants (Katsube et al., Citation2004).
Moreover, several bioactive components isolated from plant sources have been proposed for their health-promoting effects, for example grape seed proanthocyanidins have been shown to exhibit cardioprotective ability in animals and human beings (Bagchi et al., Citation2003). There is also continued interest on the application of soy isoflavones as a potential alternative to the synthetic selective estrogen receptor modulators that are currently applied in hormone replacement therapy and also in the prevention and/or delaying of atherogenesis (Cassidy et al., Citation2003; Dijsselbloem et al., Citation2004). In addition, the catechins constituents of black tea have recently been found to significantly reduce endothelial dysfunction in patients with coronary artery disease, thus suggesting that other flavonoids and polyphenolic components of tea can favorably contribute to vascular health (Widlansky et al., Citation2005). Plant-derived phenolic compounds are also accepted as chemopreventive agents as they can potentially inhibit several stages of carcinogenesis in vivo., particularly caffeic acid phenethyl ester and cucurmin, which have been found to decrease the incidence of intestinal tumors by increasing enterocyte apoptosis (Mahmoud et al., Citation2000). Although the exact role of polyphenolic compounds in nutrition needs to be unraveled, accumulated evidence increasingly suggests that these compounds can potentially be used as therapeutic alternatives and/or to complement existing therapies.
Plant phenolics have been widely described for their antioxidant activities, and the mechanism of action of these phenolic antioxidants include mainly their free radical scavenging activities, metal chelating properties, ability to regulate gene expression, and acting as co-antioxidants (Bors et al., Citation1990; Mira et al., Citation2002; Toyokuni et al., Citation2003). The activities of the extracts in the TEAC assay indicated that the phenolic-rich extracts were potent donors of hydrogen, a property inherent of phenolic compounds (Bors et al., Citation1990; Rice-Evans & Miller, Citation1996). The activity order observed in the TEAC assay is B. multiflora. > E. macrocarpum. > G. psychotroides. > O. mauritiana. > T. cordifolia. > T. rigida. > C. vaughanii. > T. coriacea. > P. terebinthina. > C. mauritianum.. The reducing potential of the plant extracts showed a similar pattern of activity as the ABTS⋅ + radical scavenging, while the hypochlorous acid scavenging activity measured is in the following decreasing order: B. multiflora. > E. macrocarpum. > C. vaughanii. ≈ O. mauritiana. > T. cordifolia. > G. psychotroides. > T. rigida. > T. coriacea. ≈ P. terebinthina. > C. mauritianum.. Literature data widely reports the free radical scavenging activities of medicinal and edible plants indicating their antioxidant efficacy and potential health benefits (Lee et al., Citation2000; Kang et al., Citation2003). A recent study on the endemic plants of Réunion island, particularly Croton mauritianus., Psidia argentea., Psidia boivinii., Psidia salaziana., and Gaertnera vaginata., has shown appreciable levels of free radical scavenging activity (Poullain et al., Citation2004). These data, along with the currently reported results, indicate the antioxidant efficacy of the Mascarene flora.
The ability of the plant extracts to protect deoxyribose sugar a measure of the ability to scavenge the hydroxyl radicals generated by the Fenton system was in the decreasing order T. cordifolia. > P. terebinthina. > O. mauritiana. ≈ T. coriacea. > C. vaughanii. > B. multiflora. > E. macrocarpum. > G. psychotroides. > T. rigida. > C. mauritianum.. In a similar system, methanol extracts of lotus plumule and lotus blossom were potent scavengers of hydroxyl radicals preventing deoxyribose degradation by 60% (Wang et al., Citation2003) The data suggests that these Mauritian endemic plants, in particular B. multiflora., E. macrocarpum., T. cordifolia., O. mauritiana., and C. Yaughanii., are good scavengers of reactive oxygen species. Several of these endemics reported in this study have traditional uses, for instance Psidia terebinthina. is used for its expectorant properties and used against boils, abscesses, fever, wounds; Tylophora coriacea. is used against vomiting, and Crinum mauritianum. and Gaertnera psychotroides. are considered as treatments for rheumatic pain () (Gurib-Fakim et al., Citation1995Citation1996aCitationb). The bioactive compounds responsible for these medicinal uses are unknown, but there are indications that the phenolic components may play an important role and that some of these medicinal properties may result from the antioxidative potentials of the phytochemicals. Several herbal medicines rich in flavonoids and phenolic compounds are viewed as promising therapeutic drugs for free radical–mediated pathologies (Lee et al., Citation2000; Kang et al., Citation2003; Soobrattee et al., Citation2005). Kang et al. (Citation2003) reported the substantive antioxidant activity of solvent extracts of traditionally used herbal medicines used in Korea with superoxide, hydroxyl radical, and lipid peroxide scavenging activities.
Lipid peroxidation, particularly of unsaturated lipids in biomembrane, disrupts their important structural and protective function, accompanying pathologic events such as chronic inflammatory processes, diabetes, atherogenesis, Alzheimer disease, and aging (Griesmacher et al., Citation1995; Reaven & Witztum, Citation1996; Rikans & Hornbrook, Citation1997; Butterfield & Lauderback, Citation2002). The plant extracts used in this study were potent inhibitors of microsome lipid peroxidation induced by AAPH suggesting that the inhibition is intrinsically linked to peroxyl radical scavenging. The hierarchy of activity was as follows: B. multiflora. > O. mauritiana. > G. psychotroides. > E. macrocarpum. > T. cordifolia. > T. rigida. > C. vaughanii. > T. coriacea. > P. terebinthina.. Plant extracts and plant-derived antioxidants are receiving wide attention in the food industry and in biomedical research primarily as a result of their ability to stabilize bulk oils, emulsions, and biological membrane against lipid peroxidation (Shahidi, Citation1997; Aruoma et al., Citation1998). Thus, B. multiflora., O. mauritiana., and G. psychotroides. can be effective lipid peroxidation inhibitors in food systems as well as prophylactic agents against biological membrane peroxidation. However, the data pertaining to the bioactive components responsible for such activity and also the cytotoxicity of the extracts will delineate the future use of these Mauritian endemic plants.
The Mauritian endemic flora acts as a reservoir of putative bioactive compounds and, therefore, shows tremendous potential for research and development. This indicates that there is need for the implementation of a sustainable development and conservation policy for Mauritius, in particular to protect the native plants from exotic invasive species. Several conservation programs have been set up worldwide to preserve the dwindling level of biodiversity, in particular the conservation of endemic and medicinal plants of the Thar Desert and the flowering plants of Peru (Khan et al., Citation2003; Van der Werff & Consiglio, Citation2004). In Mauritius, existing conservation programs need to be strengthened to protect the endemic medicinal plants and other plant species, which are potential sources of untapped bioactive components. Among the above-mentioned list of plants studied, Croton vaughanii., Tambourissa cordifolia., Tylophora coriacea., Turraea rigida., Psidia terebinthina., and Badula multiflora. are highly vulnerable species with only five individuals of Croton vaughanii. reported in the wild. Our study showed the antioxidant effectiveness of these highly endangered plant species thus suggesting that reinforcement of the existing conservation strategies will allow safeguarding of the Mauritian heritage while alternative means of regeneration of the threatened species via tissue culture can also be considered as a strategy to increase the plant population.
Acknowledgments
The authors are grateful to the Tertiary Education Commission, the University of Mauritius, and the Mauritius Research Council for financial support. Professor Aruoma is Adjunct Research Professor at the University of Mauritius.
References
- Arnold TH, de Wet BC (1993): Plants of Southern Africa: Names and distribution. Mem Bot Sur S Afr 62: 1–825.
- Aruoma OI (1994): Deoxyribose assay for detecting hydroxyl radicals. Methods Enzymol 233: 57–66.
- Aruoma OI, Deiana M, Jenner A, Halliwell B, Kaur H, Banni S, Corongiu FP, Dessi MA, Aeschbach R (1998): Effect of hydroxytyrosol found in extra virgin olive oil on oxidative DNA damage and on low density lipoprotein oxidation. J Agric Food Chem 46: 5181–5187.
- Bagchi D, Sen CK, Ray SD, Das DK, Bagchi M, Preus HG, Vinson JA (2003): Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat Res 523–524: 87–97.
- Bahorun T, Gurib-Fakim A, Aruoma OI (2003): Plant bioactive components as antioxidant prophylactic agents. In: Bahorun T, Gurib-Fakim A, eds., Molecular and Therapeutic Aspects of Redox Chemistry. London, OICA International (UK) Limited, pp. 171–189.
- Benzie IF, Strain JJ (1996): The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal Biochem 239: 70–76.
- Bors W, Heller W, Michel C, Saran M (1990): Flavonoids as antioxidants: Determination of radical scavenging efficiencies. Methods Enzymol 186: 343–355.
- Butterfield DA, Lauderback CM (2002): Lipid peroxidation and protein oxidation in Alzheimer's disease brain: Potential causes and consequences involving amyloid β.-peptide-associated free radical oxidative stress. Free Radic Biol Med 32: 1050–1060.
- Campos A, Lissi E (1997) Kinetics of the reaction between 2,2-azinobis. (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) derived radical cations and phenols. Int J Chem Kin 29: 219–224.
- Cao J, Ren LL, Liu JW, Li Y, Qu HY (2004): Gene expression spectra in human leukemia HL-60 cells treated with EGCG. Mutat Res 556: 193–200.
- Cassidy A, Teresa SP, Rimbach G (2003): Molecular mechanisms by which dietary isoflavones potentially prevent atherosclerosis. Exp Rev Mol Med 5: 1–15.
- Cowling RM, Hilton-Taylor C (1994): Patterns of plant diversity and endemism in Southern Africa: An overview. In: Huntley BJ, ed., Botanical Diversity in Southern Africa. Strelitzia, National Botanical Institute, Pretoria, pp. 31–52.
- Dijsselbloem N, Berghe WV, Naeyer AD, Haegeman G (2004): Soy isoflavone phytochemicals in interleukin-6 affections. Multipurpose nutraceuticals at the crossroad of hormone replacement, anti-cancer and anti-inflammatory therapy. Biochem Pharmacol 68: 1171–1185.
- Farombi EO (2003): African indigenous plants with chemotherapeutic potentials and biotechnological approach to the production of bioactive prophylactic agents. Afr J Biotech 2: 662–671.
- Fujiki H, Suganuma M, Kurusu M, Okabe S, Imayoshi Y, Taniguchi S, Yoshida T (2003): New TNF-α releasing inhibitors as cancer preventive agents from traditional herbal medicine and combination cancer prevention study with EGCG and sulindac or tamoxifen. Mutat Res 523–524: 119–125.
- Griesmacher A, Kindhauser M, Andert SE (1995): Enhanced serum levels of thiobarbituric-acid reactive substances in diabetes mellitus. Am J Med 98: 469–475.
- Guého J (1988): La végétation de l'île Maurice, Mauritius, Edition de l'Ocean Indien, pp. 1–86.
- Gurib-Fakim A (1991): Phytochemical screening of 38 Mauritian Medicinal Plants. Revue Agricole et Sucrière de l'île Maurice 69: 42–50.
- Gurib-Fakim A, Guého J (1995): Plantes médicinales de Maurice. Tome 1. Mauritius, Edition de l'Ocean Indien, pp. 1–485.
- Gurib-Fakim A, Guého J, Bissoondoyal MD (1996a): Plantes Médicinales de Maurice. Tome 2. Mauritius, Editions de L'Ocean Indien, pp. 1–532.
- Gurib-Fakim A, Guého J, Bissoondoyal MD (1996b): Plantes Médicinales de Maurice. Tome 3. Mauritius, Editions de L'Ocean Indien, pp. 1–472.
- Halliwell B, Gutteridge JMC, Aruoma OI (1987): The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 165: 215–219.
- Hou Z, Lambert JD, Chin KV, Yang CS (2004): Effects of tea polyphenols on signal transduction pathways related to cancer chemoprevention. Mutat Res 555: 3–19.
- Ishige K, Schubert D, Sagara Y (2001): Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med 30: 433–446.
- Ivanova D, Gerova D, Chervenkov T, Yankova T (2005): Polyphenol and antioxidant capacity of bulgarian medicinal plants. J Ethnopharmacol 96: 145–150.
- Kang DG, Yun CK, Lee HS (2003): Screening and comparison of antioxidant activity of solvent extracts of herbal medicines used in Korea. J Ethnopharmacol 87: 231–236.
- Katsube T, Abata H, Ohta Y, Yamasaki Y, Anuurad E, Shiwaku K (2004): Screening for antioxidant activity in edible plant products: Comparison of low-density lipoprotein oxidation assay, DPPH radical scavenging assay, and Folin-Ciocalteu assay. J Agric Food Chem 52: 2391–2396.
- Khan TI, Dular AK, Solomon DM (2003): Biodiversity conservation in the Thar Desert; with emphasis on endemic and medicinal plants. The Environmentalist 23: 137–144.
- Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD (2002): Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am J Med 113: 71S–88S.
- Lamaison JLC, Carnet A (1990): Teneurs en principaux flavonoides des fleurs de Crataegus monogyna Jacq et de Crataegus laevigata (Poiret D. C) en fonction de la vegetation. Pharm Acta Helvetiae 65: 315–320.
- Lee YM, Kim H, Hong E, Kang B, Kim S (2000): Water-extract of 1:1mixture of Phellodendron cortex. and Aralia cortex. has inhibitory effects on oxidative stress in kidney of diabetic rats. J Ethnopharmacol 73: 429–436.
- Levêque D, Wihlm J, Jehl F (1996): Pharmacologie des Catharanthus alcaloïdes. Bull Cancer 83: 171–186.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951): Protein measurement with the Folin-phenol reagents. J Biol Chem 193: 265–275.
- Maggs GL, Craven P, Kolberg HH (1998): Plant species richness, endemism, and genetic resources in Namibia. Biodiversity Cons 7: 435–446.
- Mahmoud NN, Carothers AM, Grunberger D, Bilinski RT, Churchill MR, Martucci C, Newmark HL, Bertagnolli MN (2000): Plant phenolics decrease intestinal tumours in an animal model of familial adenomatous polyposis. Carcinogenesis 21: 921–927.
- McGregor L, Bellangeon M, Chignier E, Lerond L, Rouselle C, McGregor JL (1999): Effect of a micronized purified flavonoid fraction on in vivo platelet functions in the rat. Thromb Res 94: 235–240.
- Mira L, Fernandez MT, Santos M, Rocha R, Florěncio MH, Jennings KR (2002): Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic Res 36: 1199–1208.
- Neergheen VS, Soobrattee MA, Bahorun T, Aruoma OI (2006): Characterization of the phenolic constituents in Mauritian endemic plants as determinants of their antioxidant activities in vitro.. J Plant Physiol 163: 787–799.
- Owuor ED, Kong AT (2002): Antioxidants and oxidants regulated signal transduction pathways. Biochem Pharmacol 64: 765–770.
- Porter LJ, Hristch LN, Chan BC (1986): The conversion of procyanidins and prodelphinidins to cyanidins and delphinidins. Phytochemistry 25: 225–230.
- Poullain C, Girard-Valenciennes E, Smadja J (2004): Plants from Réunion island: Evaluation of their free radical scavenging and antioxidant activities. J Ethnopharmacol 95: 19–26.
- Reaven PD, Witztum JL (1996): Oxidized low density lipoproteins in atherogenesis: Role of dietary modifications. Ann Rev Nutr 16: 51–71.
- Rice-Evans CA, Miller NJ (1996): Antioxidant activities of flavonoids as bioactive components of food. Biochem Soc Transac 24: 790–795.
- Rikans LE, Hornbrook KR (1997): Lipid peroxidation, antioxidant protection and aging. Biochim Biophys Acta 1362: 116–127.
- Shahidi F (1997): Natural Antioxidants: Chemistry, Health Effects and Applications, Champaign, IL, American Oil Chemist's Society Press, pp. 1–414.
- Singleton VL, Rossi JA Jr (1965): Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticul 16: 144–153.
- Škerget M, Kotnik P, Hadolin M, Hras RA, Simonic M, Knez Z (2005): Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem 89: 191–198.
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T (2005): Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat Res 579: 200–213.
- Spignoli G (2000): Protective effects of dietary flavonoids on cardiovascular system and circulation. Eur Bull Drug Res 8: 1–8.
- Toyokuni S, Tanaka T, Kawaguchi W, Lai Fang NR, Ozeki M, Akatsuka S, Hiai H, Aruoma OI, Bahorun T (2003): Effects of the phenolic contents of Mauritian endemic plant extracts on promoter activities of antioxidant enzymes. Free Radic Res 37: 1215–1224.
- Van der Werff H, Consiglio T (2004): Distribution and conservation significance of endemic species of flowering plants in Peru. Biodiversity Cons 13: 1699–1713.
- Wang L, Yen JH, Liang HL, Wu MJ (2003): Antioxidant effect of methanol extracts from lotus plumule and blossom (Nelumbo nucifera. Gertn.). J Food Drug Anal 11: 60–66.
- Weiss SJ, Klein R, Slivka A, Wei M (1982): Chlorination of taurine by the human neutrophils. J Clin Invest 70:598–607.
- Widlansky ME, Duffy SJ, Hamburg NM, Gokce N, Warden BA, Wiseman S, Keaney JF, Frei B, Vita JA (2005): Effects of black tea consumption on plasma catechins and markers of oxidative stress and inflammation in patients with coronary artery disease. Free Radic Biol Med 38: 499–506.