Abstract
Dehydroleucodine (DhL), isolated from Artemisia douglasiana. Besser (Asteraceae), prevents damage in acetic acid–induced colitis in rats. The trinitrobenzene sulfonic acid (TNBS)-induced model of chronic inflammation of the rat colon was used to determine the efficacy of DhL, at dose of 40 mg/kg, administered in enema form. All rats were sacrificed on day 7 and the colon excised, weighed, and rated macroscopically. Sections of colon were processed for light microscopy. Macroscopic scoring showed a decrease (p < 0.001) in the degree of damage in the DhL group compared with the group of rats treated with TNBS. Colon mass decreased (p < 0.05) in the DhL group compared with the TNBS group. Treatment with DhL resulted in a reduction in the incidence of diarrhea. Light-microscopic examination revealed preservation of mucosal architecture in the DhL-treated rats. DhL had an effective healing capacity at both macroscopic and microscopic levels. We conclude that DhL decreases injury and/or promotes healing in this model.
Introduction
Infusions of fresh leaves of Artemisia douglasiana. Besser (Asteraceae), popularly known as “matico,” are used in folk Argentinean medicine as a cytoprotective agent, to treat peptic ulcers, and as antispasmodic (Ariza Espinar & Bonzani Citation1992). In Mexico, aerial parts of Artemisia mexicana. Willd (Asteraceae) are use in traditional medicine for stomachache, diarrhea, intestinal infections, and wounds (Navarro et al., Citation1996). Moreover, in Korean folk medicine, Artemisia. species have been used for the treatment of chronic diarrhea (Kim et al., Citation1999).
Dehydroleucodine (DhL), a sesquiterpene lactone of the guaianolide type isolated from Artemisia douglasiana., shows a pharmacological cytoprotective effect and prevents the formation of gastric lesions induced by various necrotizing agents (Giordano et al., Citation1990; Piezzi et al., Citation1992). DhL prevented the damage and inflammation in acetic acid–induced colitis in rats and TNBS-induced colitis in mice when it was administered 1 h before colitis induction (Wendel et al., Citation1999).
Inflammatory bowel disease (IBD) is a denomination that refers essentially to two different entities known as Crohn disease and ulcerative colitis (Hanauer, Citation1993). IBD is characterized by chronic inflammation of the small and large bowel and infiltration by inflammatory leukocytes such as neutrophils, macrophages, and lymphocytes into the gastrointestinal tract. A major limitation on IBD research has been the lack of good models of this disease (Kim & Berstad, Citation1992). Of the numerous animal models of IBD, none could be said to exhibit all the features of the human condition. However, many of these models are valuable tools for the study of the pathogenesis of these disorders and for testing novel therapeutic regimens. The 2,4,6-trinitrobenzene sulfonic acid (TNBS) model has been used extensively in this regard, largely because of its simplicity and because the colitis produced by intracolonic administration of this hapten persists for a number of weeks, permitting studies involving repeated drug administration (Morris et al., Citation1989). On the other hand, the caustic properties of the TNBS-ethanol solution cause extensive acute injury in the colon. It has been suggested that this model of colitis is useful to study events that occur at the time of inflammation and repair (Yamada et al., Citation1992). In this work, we investigated the possible reparative effect of enemas of DhL on colonic ulceration and mucosal injury in rats with TNBS-induced colitis throughout a week.
Materials and Methods
Extraction and purification of DhL
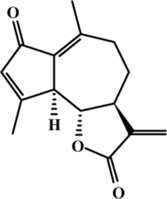
Artemisia douglasiana. Besser was collected in the mountains of the province of San Luis, Argentina, and a voucher specimen was deposited in the Herbarium of the Universidad Nacional of San Luis (UNSL no. 55). DhL was extracted as previously described (Giordano et al., Citation1990). Briefly, the air-dried material was soaked in chloroform at room temperature. The extracts were evaporated in vacuo. and dissolved in 95% ethanol. After addition of 4% aqueous lead tetraacetate solution, the aqueous cloudy solution was filtered through a celite pad, and the filtrate was concentrated under vacuum. The mixture was extracted three-times with chloroform, and the solution was concentrated under vacuum. The final residue was chromatographed in a medium-pressure chromatography system using 1:9 EtAcO/hexane as eluent. DhL (100% purity) was identified by 1H or 13C nuclear magnetic resonance, mass spectrometry, or melting point analysis, and its structure () was identical to that cited by others authors (Giordano et al., Citation1990).
Animals
Male Wistar rats, weighing 200–250 g at the start of the experiment, were used. They were maintained in a restricted access room, which was temperature controlled at 26°C. The light-dark cycle of the room was 12 h/12 h. They were provided with food and water ad libitum.. All experiments were in compliance with the ANMAT No. 6344/96 for animal care guidelines.
Induction of colitis
Colitis was induced by the intracolonic administration of 30 mg of TNBS (Sigma Chemicals, St. Louis, MO, USA) in 500 µL of 50% ethanol, as previously described (Wallace et al., Citation1989). Rats were lightly anesthetized with ether, and an 8-cm-long cannula was inserted rectally for instillation of the TNBS. The tip of the cannula reached approximately the splenic flexure of the colon. To ensure that the TNBS-ethanol solution was not immediately expelled by the rat, the cannula was left in place for 15 s prior to its removal. The rat was then allowed to recover from the anesthetic and returned to its cage.
Effects of intracolonic treatment with DhL
Rats were randomly assigned to one of three groups (at least eight rats per group). (A) DhL group: Rats received DhL (40 mg/kg) in 500 µL of 0.4% carboxymethyl cellulose (CMC) (Sigma Chemicals) intracolonically daily from day 0 to day 6, using a cannula similar to that used to instill TNBS. This concentration of DhL has proved to be the dose of choice in other experimental studies. (B) TNBS group: Rats received the vehicle (CMC) at the same times as in group A. (C) Control group (uninflamed): Rats received the vehicle at the same times as in group A and normal saline (0.9%; w/v) in place of TNBS.
Assessment of damage
One week after the induction of colitis, all rats were sacrificed by cervical dislocation, and laparatomy was performed. The distal colon was removed and opened by a longitudinal incision. The presence or absence of adhesions between the colon and other organs was recorded. Colonic damage was scored on a 0 (normal) to 10 (severe) scale, according to criteria described by Wallace et al. (Citation1989), shown in . Mucosal damage was measured macroscopically and expressed in mm2/rat. All scoring and measurements of damage were performed by one observer using a magnifying glass. For the purpose of scoring, inflammation was defined as hyperemia and thickening of the bowel wall. After scoring, the weight of 8 cm of the distal colon was recorded.
Table 1.. Criteria for macroscopic scoring of colonic ulceration and inflammation.
Gross observation
Loss of body weight and diarrhea (loose stool and/or diarrhea) are among the important clinical signs of colitis. Rats were weighed 9 AM to 10 AM before TNB administration as well as before necropsy. Diarrhea was graded according to the criteria: 0 (normal) to 3 (severe) (Satoh et al., Citation1997).
Fecal occult bleeding
Fecal occult bleeding was examined immediately after animals were sacrificed. Fecal samples were taken from the colon, with careful avoidance of contact with blood. The fecal occult bleeding was tested by SANGROCULT assay (Laboratories Britania S. A., Buenos Aires, Argentina).
Morphologic studies
From microscopic study, sections of distal colon were obtained from the same areas of the large intestine during autopsy. Samples were fixed in Bouin's fluid during 8 h, and tissue was dehydrated in a graded series of ethanol, cleared in xylene, and embedded in paraffin. Serial sections were cut at 5 µm, mounted on glass slides, and stained with hematoxylin-PAS (periodic acid–Schiff). Stained sections were examined under a light microscope and photographed with a Leitz Wetzlar photomicrographic camera (Wetzlar, Germany).
Statistics
The statistical significance of differences among means was assessed by analysis of variance (ANOVA) with multiple comparison method by Tukey-Kramer and Student's t.-test. Nonparametric data (score) were analyzed with the Mann-Whitney U.-test. A probability of p < 0.05 was considered as statistically significant.
Results
TNBS-induced colitis
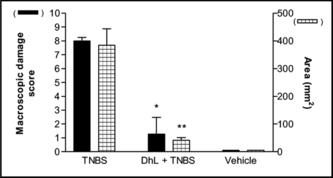
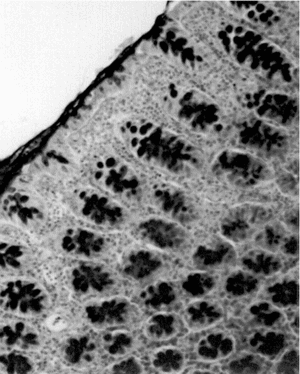
Intracolonic administration of TNBS in a vehicle of 50% ethanol produced ulceration and inflammation in the distal colon that persisted throughout the 1-week study period (). Areas of ulceration were frequently separated by macroscopically normal mucosa. Marked thickening of the bowel wall was evident. Diarrhea was observed in almost all rats receiving TNBS (TNBS group), when they were sacrificed 1 week after TNBS (). Similarly, adhesions between the distal colon and other organs, particularly the small intestine, were frequently observed in control animals.
Effects of intracolonic treatment with DhL
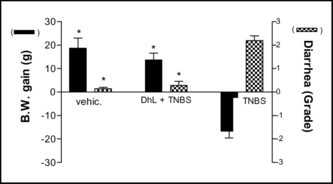
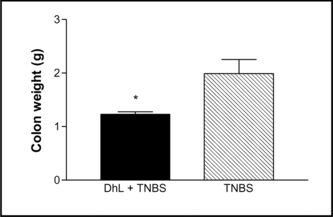
Daily administration of DhL during 7 days reduced significantly the colonic damage. Ulceration was still observed in rats treated with DhL, but the areas of involvement were markedly reduced compared with rats treated with the vehicle (). In addition to reducing the extent of colonic ulceration, treatment with DhL resulted in a reduction in the incidence of diarrhea () and the incidence of adhesions between the colon and other tissues.The wet weight of inflamed colonic tissue is considered a reliable and sensitive indicator of the severity and extent of inflammatory response (Rachmilewitz et al., Citation1989). The reduction of colonic damage provided by DhL was accompanied by significant decreases in colon weight (). Starting body weights for all treatment groups did not differ significantly. Over the 7 days of this study, the body weights of control group (uninflamed) rats increased by 18.75±4.2 g () and DhL group by 13.66±2.9 g (p < 0.001 vs. TNBS group). TNBS treatment group experienced the greatest drop in body weight on day 7 of colitis (). This matches results by Morris et al. (Citation1989), who observed an 8–10% loss in body weight in TNBS rats, mainly occurring during the first week of treatment. Hogaboam et al. (Citation1995) demonstrated that TNBS-induced colitis was associated with a markedly suppressed food intake for the first 72 h. The severity of colitis showed a close direct relationship with fecal occult bleeding, and reduction of colonic lesions was associated with decreased bleeding (Pfeiffer & Qiu, Citation1995). All rats treated with TNBS followed by vehicle exhibited fecal occult bleeding at the time of sacrifice. Treatment with DhL reduced the fecal occult bleeding. The TNBS-induced inflammation can be seen for at least 8 weeks (Morris et al., Citation1989), suggesting that the effects we observed were due to treatment with DhL per se., and not due to spontaneous healing.
Histology
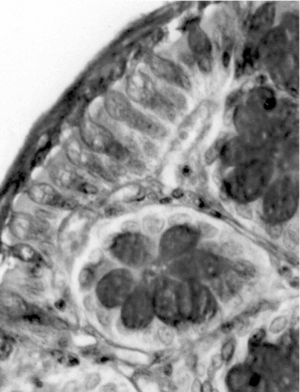
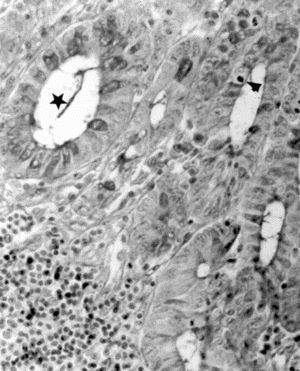
Light-microscopic examination revealed intense inflammation and extensive mucosal damage characterized by infiltration of mononuclear leukocytes (lymphocytes and macrophages) and wall thickening (). The architecture of the crypts was distorted: some crypts were fused in the apical region, and mucus appeared to have been discharged into the lumen (). The mitosis may reflect increased proliferation as a mechanism of tissue repair in response to TNBS (). Mucosal injury 1 week after exposure to TNBS was variable: in some regions of the bowel, the epithelium remained intact, whereas in others the epithelium had disappeared with features of ulceration and desquamation. In animals treated with DhL, there was a preservation of mucosal architecture, with well-preserved goblet cells and little cellular infiltration in the muscularis mucosa. The PAS-positive reaction was present in DhL group in crypts and along the epithelial surface (). The preparations showed a thin mucus layer (PAS +) covering the surface (). No damage was observed in the colon from animals treated with only vehicle (not shown).
Figure 5 Micrograph of rat colon mucosa showing dilated and fused crypts with intraluminal mucus content (arrowhead) in TNBS-group. Note PMN and lymphocyte infiltration. PAS-hematoxylin, × 100.
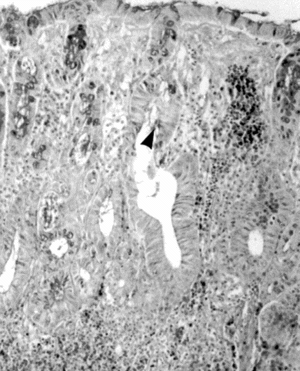
Figure 6 Micrograph of rat colon mucosa of TNBS group. Dilated crypts (★); mitosis (arrowhead). PAS-hematoxylin, × 250.
Discussion
The current results confirmed that intracolonic administration of the hapten, TNBS, combined with the mucosal barrier breaker, 50% ethanol, results in long-lasting ulceration and inflammation of the rat colon. This prolonged inflammation, which resembles human IBD, provides a suitable model with some advantages over other experimental colitis models (Morris et al., Citation1989). Indeed, the TNBS-treated rat has been one of the most intensively studied animal models of colitis for the past several years and has been associated with a dysfunction of T cell–mediated immunity and the generation of oxygen radicals (Grisham et al., Citation1991; Kim & Berstad, Citation1992).
We report here the healing processes of chronic colitis, observed with intracolonic administration of DhL, determined macroscopically and by means of light microscopy. As well as reducing the colonic damage scores, DhL reduced the incidence of diarrhea and adhesions. The latter may have been a consequence of the accelerated healing of ulcers, as we have noted a close correlation between the site of colonic ulceration and the site of adhesion formation. On the other hand, induction of colitis with TNBS markedly reduced the weight gain over the following week. DhL treatment after induction of colitis significantly increased the rate of weight gain, although not back to the levels observed in rats that did not have colitis.
The mechanism by which DhL accelerates healing is not clear, but a number of possibilities can be considered. Maintenance of the normal physiologic function of the gastrointestinal mucosa is continually compromised by the harsh extracellular environment of the gut. As a result, there are sophisticated reparative mechanisms that have the capacity to rapidly restore mucosal integrity. These include restitution, in which local epithelial cells migrate to cover denuded areas, as well as increased mucus production and blood flow, followed by proliferation and differentiation programs. The mucus serves to buffer cells against abrasion by luminal contents and to create a microclimate near the epithelial surface (Halm & Halm, Citation2000). In colon from guinea pigs with experimental colitis, the depletion of mucus was due to less mucus in goblet cells rather than a decrease in goblet cell number (Kaftan & Wright, Citation1989). In addition to mucus depletion, the composition of colonic mucus is altered in ulcerative colitis (Podolsky et al., Citation1991).
Histologic evidence clearly shows that DhL accelerates healing, and this effect would be related to the ability of the drug to stimulate mucus production. This result is consistent with the significant increase in glycoprotein synthetic activity found in a previous study when rats were treated with DhL (Guardia et al., Citation1994). Furthermore, there was an increase in mucus production in the colon in acetic acid–induced colitis rats, through treatment with DhL (Wendel et al., Citation1999). Penissi et al. (1998) have shown that DhL prevents the depletion of a mucosal protective factor, dopamine, and the release of an inflammatory mediator, 5-hydroxytryptamine provoked by ethanol in stomach and duodenum. Also, they proposed that the abundant mucoid blanket secreted after treatment with DhL acts as a diffusion barrier against ethanol.
It has been suggested that radicals and other reactive oxygen species could play an initiating role in some types of IBD, triggering the onset of the inflammation that is characteristic of the disease (Harris et al., Citation1992). Grisham et al. (Citation1991) have shown that the rat colon as well as rat colonocytes are capable of metabolizing TNBS to produce large quantities of reactive oxygen metabolites such as superoxide, hydrogen peroxide, and hydroxyl radical in vitro.. The effect of DhL on TNBS-induced colitis could be due to its antioxidant capacity (María et al., Citation2000).
An alternative explanation for the action of DhL on the TNBS model may relate to the anti-inflammatory effect. Previous findings in our laboratory have shown that DhL may prevent or reverse the inflammation process in rats. DhL inhibited both chronic and acute adjuvant carrageenan-induced inflammation phases, being most effective in the chronic phase (Guardia et al., Citation2003). Also, DhL could act as a “cell stabilizer” by inhibiting the degranulation of cells containing monoamines (Penissi et al., Citation1998). An anti-inflammatory effect has previously been observed in other sesquiterpene lactones (Hall et al., Citation1979). Recently, several Asteraceae (Artemisia ludoviciana. subsp. mexicana., Polymia maculata.) have been identified as potent inhibitors of the transcription factor NF-κB, a central mediator of the inflammatory response (Bork et al., Citation1997). Extracts from Mexican Indian medicinal plants used in traditional indigenous medicine for inflammation treatment contain sesquiterpene lactones, which specifically inhibit transcription factor NF-κB. Recent studies have implicated NF-κB in the pathogenesis of TNBS-induced colitis (Neurath et al., Citation1996). Compounds that block nuclear factor NF-κB activation have protective effects on animal model colitis (Conner et al., Citation1998), suggesting that blockade of this transcription factor may represent a valuable strategy for human IBD. Steroid-induced healing of colonic inflammation was associated with disappearance of NF-κB from extracts in patients with IBD (Ardite et al., Citation1998). Several other compounds have been reported to reduce TNBS-induced colitis in the rat model. These compounds include heparin (Fries et al., Citation1998) and pentoxifylline (Peterson & Davey, Citation1997). In both instances, the proposed mechanism of action relates to an anti-inflammatory effect. Band-shift experiments with total HeLa cell proteins revealed that DhL prevents NF-κB induction (Wendel et al., Citation2002). The anti-inflammatory effect of DhL could be due to the inhibition of transcription factor NF-κB.
Inhibition of neutrophil infiltration by a monoclonal antibody to adhesion molecules expressed on granulocytes has been shown to prevent the development of colonic ulcers and promote rapid ulcer healing in a rabbit model of TNBS colitis (Wallace et al., Citation1992). Sesquiterpene lactones containing α-methylene-γ-lactone ring successfully inhibited polymorphonuclear neutrophil chemotaxic migration (Hall et al., Citation1980). Chronic experimental intestinal inflammation, such as revealed in chronic TNBs-induced colitis, is characterized by activated T cells. Sesquiterpene lactones suppressed T-cell delayed hypersensitivity with both methylated bovine albumin and chicken egg albumin. T-lymphocyte production seemed to be depressed with helenalin having the best effects (Hall et al., Citation1979). It is also possible that DhL may act by the above-mentioned mechanisms, but the effect of DhL on inhibition of neutrophil infiltration and T-cell delayed hypersensitivity has yet to be determined.
In summary, the current study shows that DhL significantly accelerates the healing of colonic damage induced by TNBS, reduces the incidence of adhesions and diarrhea, and this activity could be due, at least in part, to its capacity to scavenge oxygen free radicals, stimulate mucus production, and to an anti-inflammatory effect. The results reported herein suggest the need to carry on further studies to explore the use of this compound in the treatment of IBD.
Acknowledgment
This work was supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Universidad Nacional de San Luis (CYTED), project no. 8504.
References
- Ardite E, Panes J, Miranda M, Salas A, Elizalde JI, Sans M, Arce Y, Bordas JM, Fernández-Checa JC, Pique JM (1998): Effects of steroid treatment on activation of nuclear factor kappaB in patients with inflammatory bowel disease. Br J Pharmacol 124: 4311–4313.
- Ariza Espinar L, Bonzani N (1992): El mático de la región de Cuyo (Argentina). Acta Farm Bonaerense 11: 39–145.
- Bork PM, Lienhard Schmitz M, Kuhnt M, Escher C, Heinrich M (1997): Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-κB. FEBS Lett 402: 85–90.
- Conner EM, Brand S, Grisham MB (1998): Inhibition of chronic granulomatous colitis by a selective proteasome inhibitor: Antagonist of nuclear transcription factor KB (NF-κB) activation. Br J Pharmacol 124: 431–433.
- Fries W, Pagiaro E, Canova E, Carraro P, Gasparini G, Pomerri F, Martin A, Carlotto C, Mazzon E, Sturniolo G, Longo G (1998): The effect of heparin on trinitrobenzene sulfonic acid induced colitis in the rat. Aliment Pharmacol Ther 12: 229–236.
- Giordano OS, Guerreiro E, Pestchanker MJ, Guzmán JA, Pastor D, Guardia T (1990): The gastric citoprotective effect of several sesquiterpene lactones. J Nat Prod 53: 803–809.
- Grisham MB, Volkmer C, Tso P, Yamada T (1991): Metabolism of trinitrobenzene sulfonic acid by the rat colon produces reactive oxygen species. Gastroenterology 101: 540–547.
- Guardia T, Guzmán JA, Pestchanker M, Guerreiro E, Giordano O (1994): Mucus synthesis and sulfhidryle groups in cytoprotection mediates by dehydroleucodine, a sesquiterpene lactone. J Nat Prod 57: 507–509.
- Guardia T, Juárez AO, Guerreiro E, Guzmán JA, Pelzer LE (2003): Anti-inflammatory activity and effect on gastric secretion of dehydroleucodine isolated from Artemisia douglasiana.. J Ethnopharmacol 88: 195–198.
- Hall IH, Lee KH, Starnes CO, Sumida Y, Wu RY, Waddell TG, Cochran JW, Gerhart KG (1979): Anti-inflammatory activity of sesquiterpene lactones and related compounds. J Pharm Sci 68: 537–541.
- Hall IH, Starnes CO, Lee KH, Waddell TG (1980): Mode of action of sesquiterpene lactones as anti-inflammatory agents. J Pharm Sci 69: 537–543.
- Halm D, Halm ST (2000): Secretagogue response of goblet cells and columnar cells in human colonic crypts. Am J Physiol Cell Physiol 278: C212–C233.
- Hanauer S (1993): Medical therapy of ulcerative colitis. Lancet 342: 412–417.
- Harris ML, Schiller HJ, Reilly PM, Donowitz M, Grisham MB, Bulkey GB (1992): Free radicals and other reactive oxygen metabolites in inflammatory bowel disease: Cause, consequence or epiphenomenon? Pharmacol Ther 53: 375–408.
- Hogaboam CM, Jacobson K, Collins SM, Blennerhassett MG (1995): The selective beneficial effects of nitric oxide inhibition in experimental colitis. Am J Physiol 268: G673–G684.
- Kaftan SM, Wright NA (1989): Studies on the mechanism of mucous cell depletion in ulcerative colitis. J Pathol 159: 75–85.
- Kim HS, Berstad A (1992): The experimental colitis in animal models. Scand J Gastroenterol 27: 529–537.
- Kim YS, Son MW, Ko JI, Cho HO, Yoo MH, Kim WB, Song IS, Kim CY (1999): Effect of DA-6034, a derivative of flavonoid, on experimental animal models of inflammatory bowel disease. Arch Pharm Res 22: 354–360.
- María AOM, Repetto M, Llesuy S, Giordano O, Guzmán JA, Guerreiro E (2000): Antioxidant activity of extract and dehydroleucodine from Artemisia douglasiana. Besser. Phytother Res 14: 558–560.
- Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk ZMR, Wallace JL (1989): A hapten-induced model of chronic inflammation and ulceration in rat colon. Gastroenterology 96: 795–803.
- Navarro V, Villareal M, Rojas G, Lozoya X (1996): Antimicrobial evaluation of some plants used in Mexican traditional medicine for the treatment of infectious diseases. J Ethnopharmacol 52: 143–147.
- Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W (1996): Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nature Med 2: 998–1004.
- Penissi AB, Fogal TH, Guzmán JA, Piezzi RS (1998): Gastroduodenal mucosal protection induced by dehydroleucodine. Mucus secretion and role of monoamines. Dig Dis Sci 43: 791–798.
- Peterson TC, Davey K (1997): Effect of acute pentoxifylline in an experimental model of colitis. Aliment Pharmacol Ther 11: 575–580.
- Pfeiffer CJ, Qiu BS (1995): Effects of chronic nitric oxide synthase inhibition on TNB-induced colitis in rats. J Pharm Pharmacol 47: 827–832.
- Piezzi R, Guzmán J, Guardia T, Pestchanker M, Guerreiro O, Giordano O (1992): Dehydroleucodine prevents ethanol-induced necrosis in the rat gastric mucosa. A histological study. Micr Elect Biol Cel 16: 45–56.
- Podolsky DK (1991): Inflammatory bowel disease. N Engl J Med 325: 928–937.
- Rachmilewitz D, Simon PL, Schwartz LW, Don Griswold E, Fondacaro JD, Wassermann MA (1989): Inflammatory mediators of experimental colitis in rats. Gastroenterology 97: 326–337.
- Satoh H, Sato F, Takami K, Szabo S (1997): New ulcerative colitis model induced by sulphidryl blockers in rats and effects of antiinflammatory drugs on the colitis. Jpn J Pharmacol 73: 299–309.
- Wallace JL, Braquet P, Ibbotson GC, MacNaughton WK, Cirino G (1989): Assessment of the role of platelet activating factor in an animal model of inflammatory bowel disease. J Lipid Med 1: 13–23.
- Wallace JL, Higa A, Mc Knight GW, Mc Intyre DE (1992): Prevention and reversal of experimental colitis by a monoclonal antibody which inhibits leukocyte adherence. Inflammation 16: 343–354.
- Wendel GH, Bocco J, Bidinost C, Guzmán JA, Giordano O, Pelzer L (2002): Study of dehydroleucodine on their anti-inflammatory activity using the transcription factor NF-κB as target. Biocell 26: 403.
- Wendel GH, Maria AOM, Mohamed F, Domínguez S, Scardapane L, Giordano O, Guerreiro E, Guzmán JA (1999): Effect of dehydroleucodine in experimental olitis in rats and mice. Pharm Res 40: 339–344.
- Yamada Y, Marshall S, Specian RD, Grisham MB (1992): A comparative analysis of two models of colitis in rats. Gastroenterology 102: 1524–1534.